Endocrinol Metab.
2024 Oct;39(5):767-776. 10.3803/EnM.2024.1952.
In Vitro Investigation of HIF-1α as a Therapeutic Target for Thyroid-Associated Ophthalmopathy
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Pathology, Institute of Hansen’s Disease, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Cheongju St. Mary’s Hospital, Cheongju, Korea
- 6Department of Ophthalmology and Visual Science, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2560285
- DOI: http://doi.org/10.3803/EnM.2024.1952
Abstract
- Background
Thyroid-associated ophthalmopathy (TAO) involves tissue expansion and inflammation, potentially causing a hypoxic microenvironment. Hypoxia-inducible factor (HIF)-1α is crucial in fibrosis and adipogenesis, which are observed in TAO progression. We investigated the effects of hypoxia on orbital fibroblasts (OFs) in TAO, focusing on the role of HIF-1α in TAO progression.
Methods
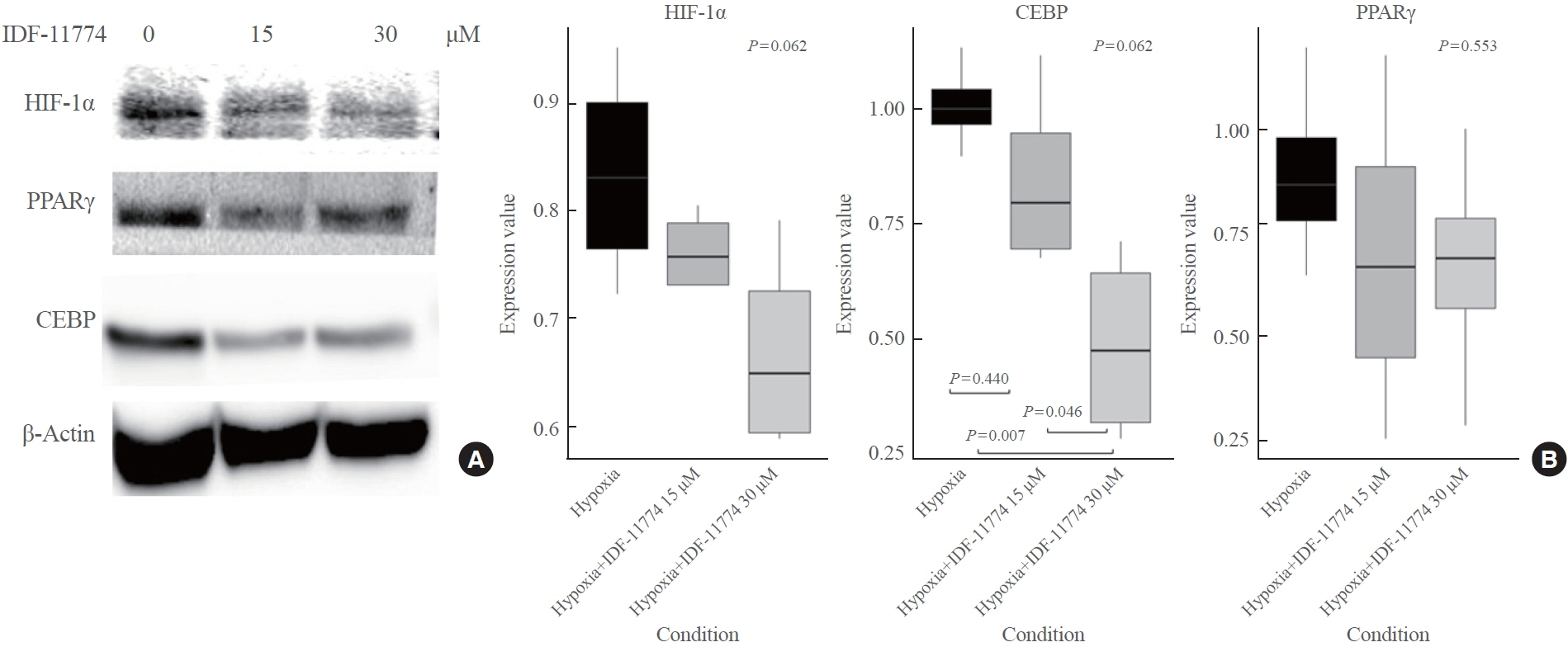
OFs were isolated from TAO and non-TAO patients (as controls). In addition to HIF-1α, adipogenic differentiation markers including peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein (CEBP) were measured by Western blot, and phenotype changes were evaluated by Oil Red O staining under both normoxia and hypoxia. To elucidate the effect of HIF-1α inhibition, protein expression changes after HIF-1α inhibitor treatment were evaluated under normoxia and hypoxia.
Results
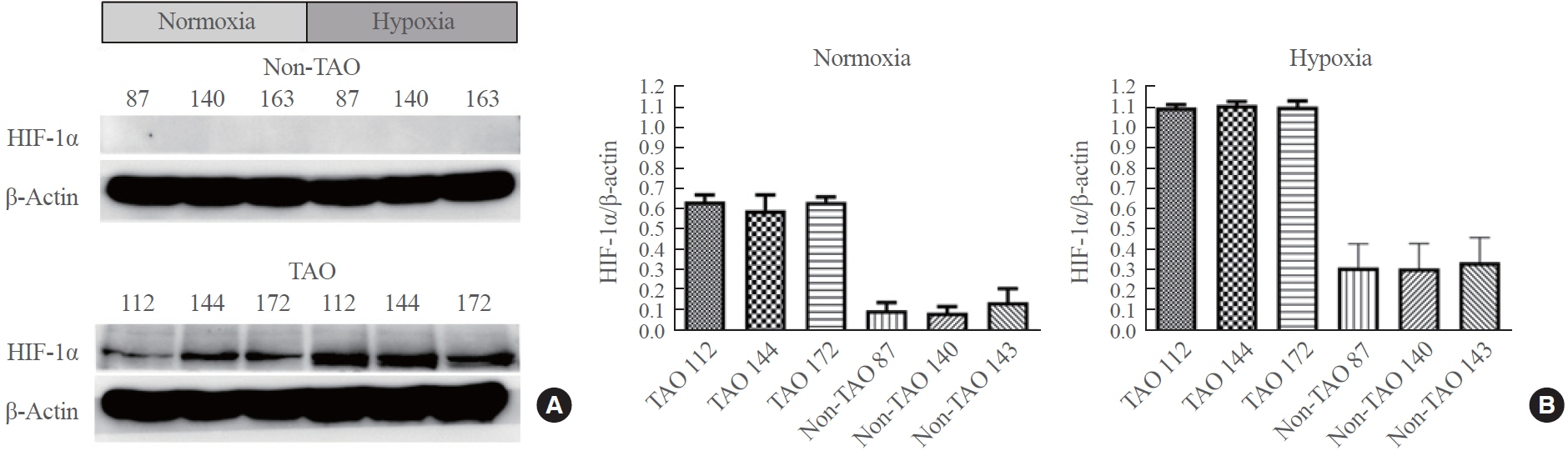
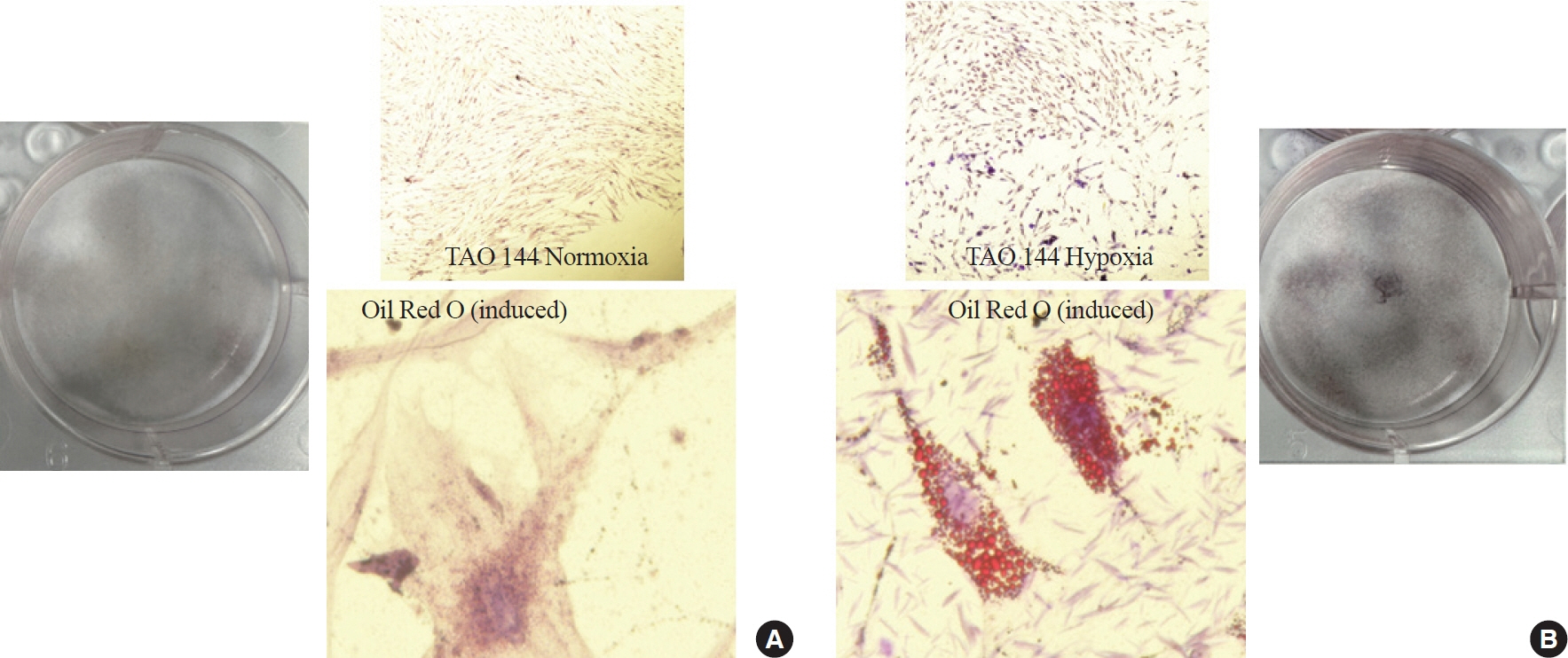
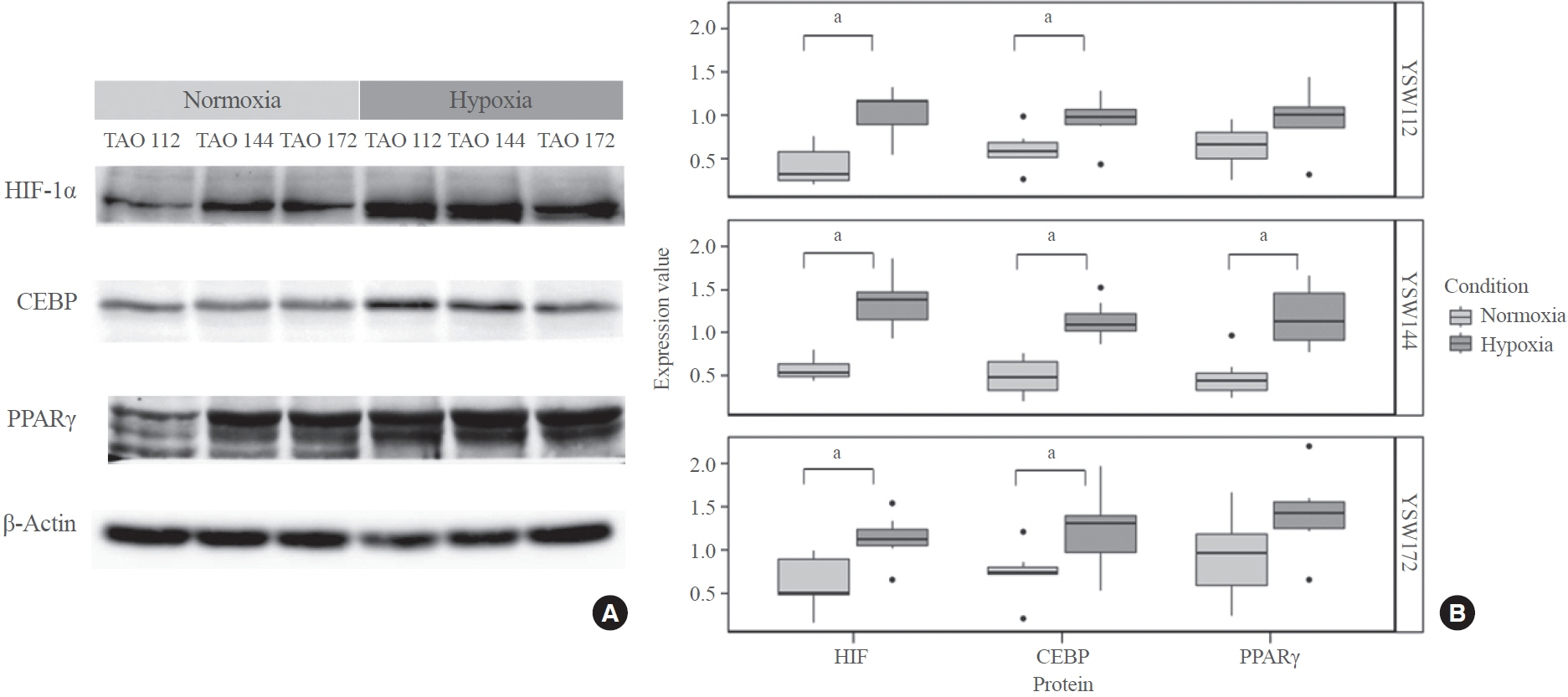
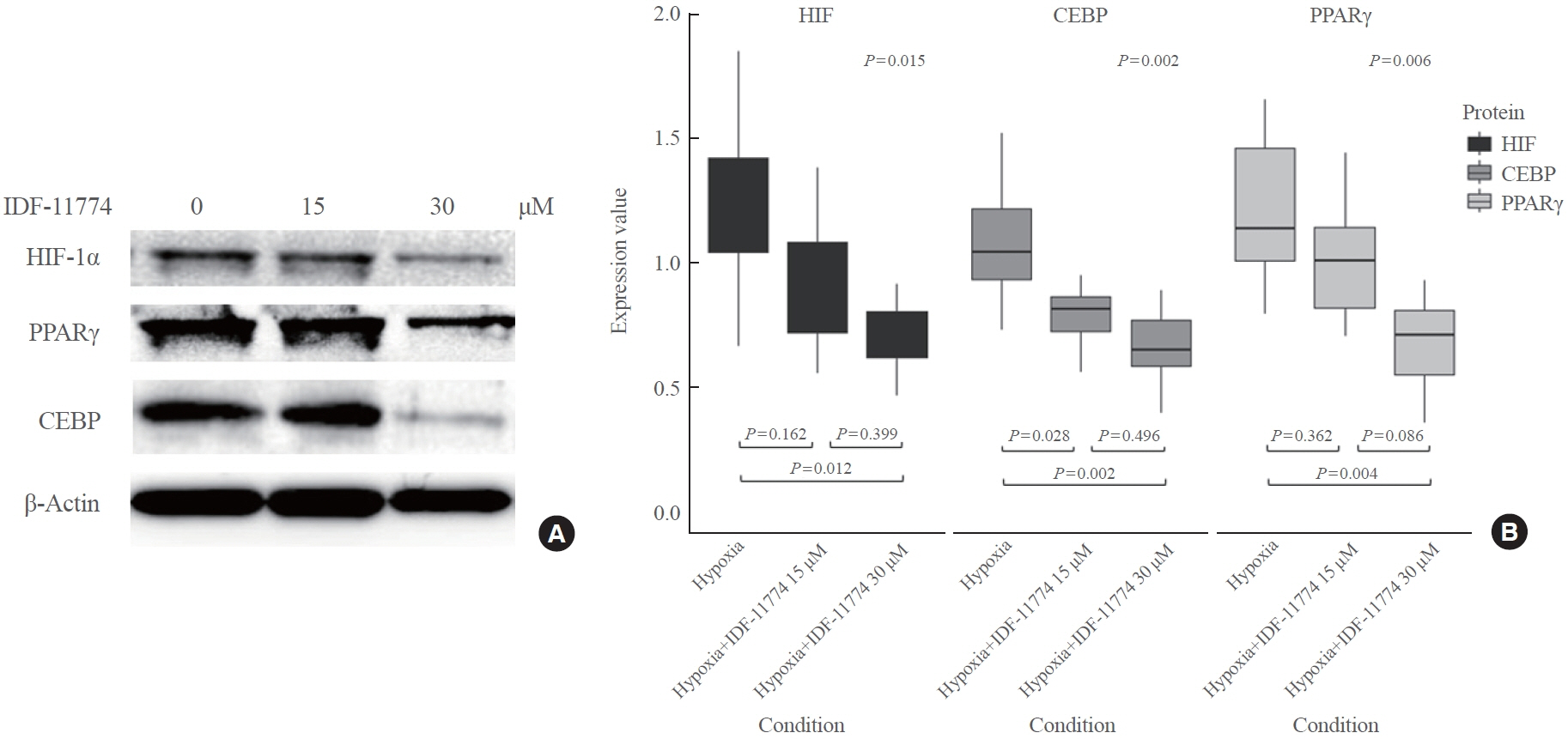
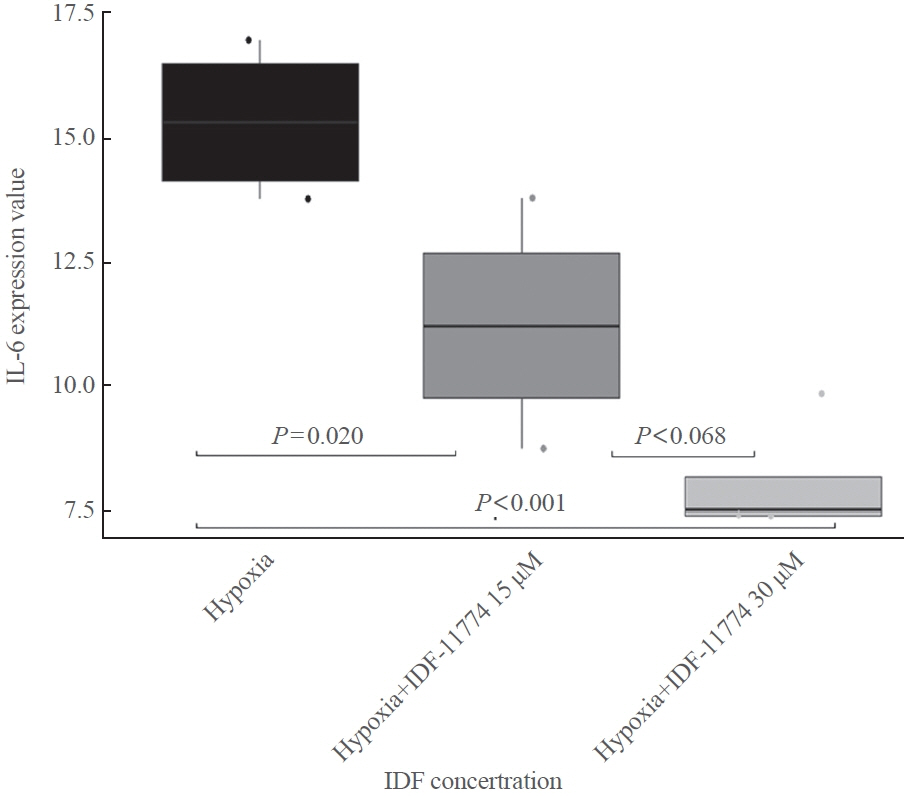
TAO OFs exhibited significantly higher HIF-1α expression than non-TAO OFs, and the difference was more distinct under hypoxia than under normoxia. Oil Red O staining showed that adipogenic differentiation of TAO OFs was prominent under hypoxia. Hypoxic conditions increased the expression of adipogenic markers, namely PPARγ and CEBP, as well as HIF-1α in TAO OFs. Interleukin 6 levels also increased in response to hypoxia. The effect of hypoxia on adipogenesis was reduced at the protein level after HIF-1α inhibitor treatment, and this inhibitory effect was sustained even with IGF-1 stimulation in addition to hypoxia.
Conclusion
Hypoxia induces tissue remodeling in TAO by stimulating adipogenesis through HIF-1α activation. These data could provide insights into new treatment strategies and the mechanisms of adipose tissue remodeling in TAO.
Figure
Reference
-
1. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010; 362:726–38.
Article2. Smith TJ, Hegedus L. Graves’ disease. N Engl J Med. 2016; 375:1552–65.
Article3. Lacheta D, Miskiewicz P, Gluszko A, Nowicka G, Struga M, Kantor I, et al. Immunological aspects of Graves’ ophthalmopathy. Biomed Res Int. 2019; 2019:7453260.4. Smith TJ, Janssen JA. Insulin-like growth factor-I receptor and thyroid-associated ophthalmopathy. Endocr Rev. 2019; 40:236–67.
Article5. Metcalfe RA, Weetman AP. Stimulation of extraocular muscle fibroblasts by cytokines and hypoxia: possible role in thyroid-associated ophthalmopathy. Clin Endocrinol (Oxf). 1994; 40:67–72.
Article6. Chng CL, Lai OF, Chew CS, Peh YP, Fook-Chong SM, Seah LL, et al. Hypoxia increases adipogenesis and affects adipocytokine production in orbital fibroblasts: a possible explanation of the link between smoking and Graves’ ophthalmopathy. Int J Ophthalmol. 2014; 7:403–7.7. Gortz GE, Horstmann M, Aniol B, Reyes BD, Fandrey J, Eckstein A, et al. Hypoxia-dependent HIF-1 activation impacts on tissue remodeling in Graves’ ophthalmopathy: implications for smoking. J Clin Endocrinol Metab. 2016; 101:4834–42.8. Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011; 364:656–65.
Article9. Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013; 93:1–21.
Article10. Dutton JJ. Anatomic considerations in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018; 34(4S Suppl 1):S7–12.
Article11. Walasik-Szemplinska D, Pauk-Domanska M, Sanocka U, Sudol-Szopinska I. Doppler imaging of orbital vessels in the assessment of the activity and severity of thyroid-associated orbitopathy. J Ultrason. 2015; 15:388–97.
Article12. Lee K, Kang JE, Park SK, Jin Y, Chung KS, Kim HM, et al. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1alpha via upregulation of VHL in a colon cancer cell line. Biochem Pharmacol. 2010; 80:982–9.13. Lee K, Ban HS, Naik R, Hong YS, Son S, Kim BK, et al. Identification of malate dehydrogenase 2 as a target protein of the HIF-1 inhibitor LW6 using chemical probes. Angew Chem Int Ed Engl. 2013; 52:10286–9.14. Kim MH, Lee TH, Lee JS, Lim DJ, Lee PC. Hif-1α inhibitors could successfully inhibit the progression of differentiated thyroid cancer in vitro. Pharmaceuticals (Basel). 2020; 13:208.
Article15. Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006; 70:1469–80.
Article16. Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012; 148:399–408.
Article17. Liu ZZ, Tian YF, Wu H, Ouyang SY, Kuang WL. LncRNA H19 promotes glioma angiogenesis through miR-138/HIF-1α/VEGF axis. Neoplasma. 2020; 67:111–8.
Article18. Befani C, Liakos P. The role of hypoxia-inducible factor-2 alpha in angiogenesis. J Cell Physiol. 2018; 233:9087–98.
Article19. Luo L, Luo G, Fang Q, Sun Z. Stable expression of hypoxiainducible factor-1α in human renal proximal tubular epithelial cells promotes epithelial to mesenchymal transition. Transplant Proc. 2014; 46:130–4.
Article20. Palsson-McDermott EM, O’Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays. 2013; 35:965–73.
Article21. Xie Y, Shi X, Sheng K, Han G, Li W, Zhao Q, et al. PI3K/ Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (review). Mol Med Rep. 2019; 19:783–91.22. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003; 3:721–32.
Article23. Hammond EM, Giaccia AJ. The role of p53 in hypoxia-induced apoptosis. Biochem Biophys Res Commun. 2005; 331:718–25.
Article24. Dong S, Liang S, Cheng Z, Zhang X, Luo L, Li L, et al. ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1αinduced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J Exp Clin Cancer Res. 2022; 41:15.
Article25. Sharma A, Sinha S, Shrivastava N. Therapeutic targeting hypoxia-inducible factor (HIF-1) in cancer: cutting gordian knot of cancer cell metabolism. Front Genet. 2022; 13:849040.
Article26. Abdi Sarabi M, Shiri A, Aghapour M, Reichardt C, Brandt S, Mertens PR, et al. Normoxic HIF-1α stabilization caused by local inflammatory factors and its consequences in human coronary artery endothelial cells. Cells. 2022; 11:3878.
Article27. Ruan H, Zhang Q, Zhang YP, Li SS, Ran X. Unraveling the role of HIF-1α in sepsis: from pathophysiology to potential therapeutics-a narrative review. Crit Care. 2024; 28:100.
Article28. Smith TJ, Janssen JA. Building the case for insulin-like growth factor receptor-I involvement in thyroid-associated ophthalmopathy. Front Endocrinol (Lausanne). 2017; 7:167.
Article29. Gortz GE, Philipp S, Bruderek K, Jesenek C, Horstmann M, Henning Y, et al. Macrophage-orbital fibroblast interaction and hypoxia promote inflammation and adipogenesis in Graves’ orbitopathy. Endocrinology. 2022; 164:bqac203.30. Van Regemorter E, Joris V, Van Regemorter V, Marique L, Behets C, Lengele B, et al. Downregulation of caveolin-1 and upregulation of deiodinase 3, associated with hypoxia-inducible factor-1α increase, are involved in the oxidative stress of Graves’ orbital adipocytes. Thyroid. 2021; 31:627–37.
Article31. Gao J, Zhu J, Zhao Y, Gan X, Yu H. Leptin attenuates hypoxia-induced apoptosis in human periodontal ligament cells via the reactive oxygen species-hypoxia-inducible factor-1α pathway. Exp Physiol. 2021; 106:1752–61.
Article32. Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012; 81:715–36.
Article33. Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-gamma (PPARgamma), and thyrotropin receptor by PPARgamma agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2002; 87:2352–8.34. Westra J, Brouwer E, Bos R, Posthumus MD, Doornbos-van der Meer B, Kallenberg CG, et al. Regulation of cytokine-induced HIF-1alpha expression in rheumatoid synovial fibroblasts. Ann N Y Acad Sci. 2007; 1108:340–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Myocardin Reverses Hypoxia-Inducible Factor-1α Mediated Phenotypic Modulation of Corpus Cavernosum Smooth Muscle Cells in Hypoxia Induced by Cobalt Chloride
- Hypoxia-Inducible Factor-1 Alpha Stabilization in Human Macrophages during Leishmania major Infection Is Impaired by Parasite Virulence
- ACY-241, a histone deacetylase 6 inhibitor, suppresses the epithelial–mesenchymal transition in lung cancer cells by downregulating hypoxia-inducible factor-1 alpha
- Is Biochemical Follow Up Possible in Peripheral Arterial Disease Treatment: Hypoxia Inducible Factor-1 Alpha?
- Vitexin Inhibits Gastric Cancer Growth and Metastasis through HMGB1-mediated Inactivation of the PI3K/ AKT/mTOR/HIF-1α Signaling Pathway