Endocrinol Metab.
2024 Oct;39(5):722-731. 10.3803/EnM.2024.1995.
Study Design and Protocol for a Randomized Controlled Trial to Assess Long-Term Efficacy and Safety of a Triple Combination of Ezetimibe, Fenofibrate, and Moderate-Intensity Statin in Patients with Type 2 Diabetes and Modifiable Cardiovascular Risk Factors (ENSEMBLE)
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 2Department of Biostatistics, Korea University College of Medicine, Seoul, Korea
- 3Department of Endocrinology and Metabolism, Kyung Hee University Hospital, College of Medicine, Kyung Hee University, Seoul, Korea
- 4Department of Internal Medicine, Hallym University Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Kyung Hee University Hospital at Gangdong, College of Medicine, Kyung Hee University, Seoul, Korea
- 6Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 7Division of Endocrinology, Department of Internal Medicine, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea
- 8Division of Endocrinology and Metabolism, Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea
- 9Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea
- 10Department of Internal Medicine, Daejeon Eulji Medical Center, Eulji University, Daejeon, Korea
- 11Division of Endocrinology and Metabolism, Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, Korea
- 12Department of Internal Medicine, Pusan National University Hospital, Busan, Korea
- 13Department of Internal Medicine, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- 14Department of Internal Medicine, Bucheon Sejong Hospital, Bucheon, Korea
- 15Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 16Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 17Department of Internal Medicine, Research Institute of Metabolism and Inflammation, Yonsei University Wonju College of Medicine, Wonju, Korea
- 18Department of Internal Medicine, Gachon University College of Medicine, Incheon, Korea
- 19Division of Endocrinology and Metabolism, Department of Internal Medicine, Research Institute of Clinical Medicine of Jeonbuk National University Medical School-Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju, Korea
- 20Division of Endocrinology, Department of Internal Medicine, Myongji Hospital, Goyang, Korea
- 21Division of Endocrinology and Metabolism, Department of Internal Medicine, Jeju National University College of Medicine, Jeju, Korea
- 22Division of Endocrinology and Metabolism, Chosun University College of Medicine, Gwangju, Korea
- 23Division of Endocrinology and Metabolism, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 24Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- 25Department of Internal Medicine, Inje University College of Medicine, Busan, Korea
- 26Department of Endocrinology, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea
- 27Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- 28Department of Internal Medicine, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 29Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 30Division of Endocrinology and Metabolism, Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea
- 31Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea
- 32Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 33Department of Endocrinology and Metabolism, Inha University College of Medicine, Incheon, Korea
- 34Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 35Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea
- 36Big Data Steering Department, National Health Insurance Service, Wonju, Korea
- 37Department of Internal Medicine, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 38Division of Endocrinology and Metabolism, Department of Internal Medicine, Dong-A University Medical Center, Dong-A University College of Medicine, Busan, Korea
- 39Division of Endocrinology and Metabolism, Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- 40Division of Endocrinology and Metabolism, College of Medicine, Hallym University, Chuncheon, Korea
- 41Department of Endocrinology and Metabolism, Konyang University Hospital, Konyang University College of Medicine, Daejeon, Korea
- 42Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
- 43Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea
- 44Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, Korea
- KMID: 2560281
- DOI: http://doi.org/10.3803/EnM.2024.1995
Abstract
- Background
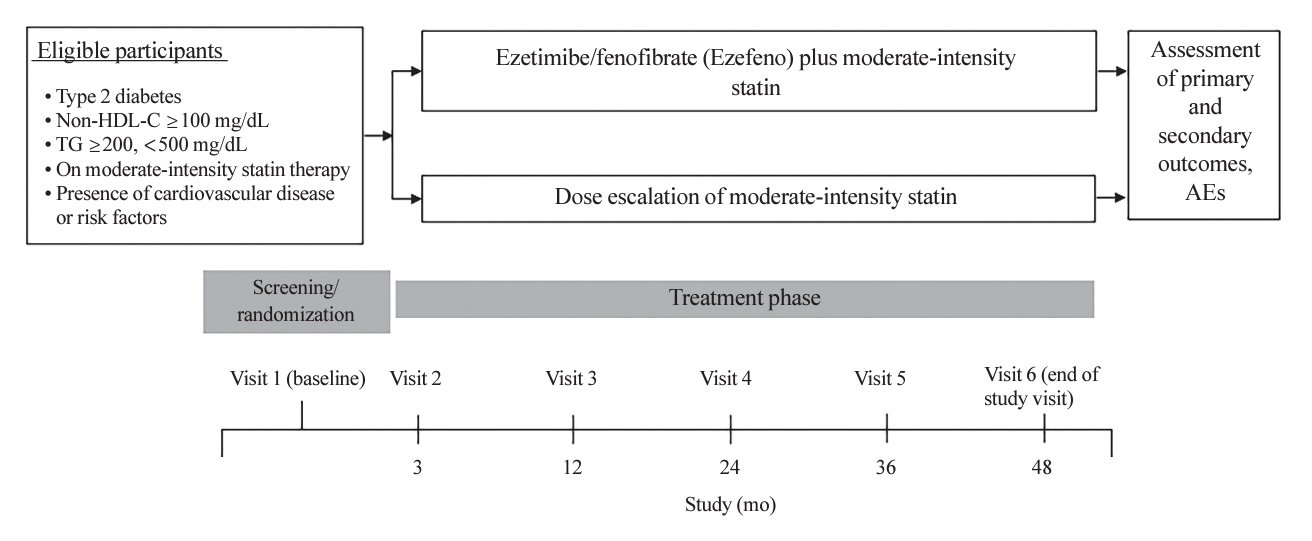
Atherogenic dyslipidemia, which is frequently associated with type 2 diabetes (T2D) and insulin resistance, contributes to the development of vascular complications. Statin therapy is the primary approach to dyslipidemia management in T2D, however, the role of non-statin therapy remains unclear. Ezetimibe reduces cholesterol burden by inhibiting intestinal cholesterol absorption. Fibrates lower triglyceride levels and increase high-density lipoprotein cholesterol (HDL-C) levels via peroxisome proliferator- activated receptor alpha agonism. Therefore, when combined, these drugs effectively lower non-HDL-C levels. Despite this, few clinical trials have specifically targeted non-HDL-C, and the efficacy of triple combination therapies, including statins, ezetimibe, and fibrates, has yet to be determined.
Methods
This is a multicenter, prospective, randomized, open-label, active-comparator controlled trial involving 3,958 eligible participants with T2D, cardiovascular risk factors, and elevated non-HDL-C (≥100 mg/dL). Participants, already on moderate-intensity statins, will be randomly assigned to either Ezefeno (ezetimibe/fenofibrate) addition or statin dose-escalation. The primary end point is the development of a composite of major adverse cardiovascular and diabetic microvascular events over 48 months.
Conclusion
This trial aims to assess whether combining statins, ezetimibe, and fenofibrate is as effective as, or possibly superior to, statin monotherapy intensification in lowering cardiovascular and microvascular disease risk for patients with T2D. This could propose a novel therapeutic approach for managing dyslipidemia in T2D.
Keyword
Figure
Reference
-
1. DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015; 1:15019.
Article2. Goldberg IJ. Clinical review 124: diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab. 2001; 86:965–71.3. Eid S, Sas KM, Abcouwer SF, Feldman EL, Gardner TW, Pennathur S, et al. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019; 62:1539–49.
Article4. Verbeek R, Hovingh GK, Boekholdt SM. Non-high-density lipoprotein cholesterol: current status as cardiovascular marker. Curr Opin Lipidol. 2015; 26:502–10.5. Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011; 4:337–45.
Article6. van Deventer HE, Miller WG, Myers GL, Sakurabayashi I, Bachmann LM, Caudill SP, et al. Non-HDL cholesterol shows improved accuracy for cardiovascular risk score classification compared to direct or calculated LDL cholesterol in a dyslipidemic population. Clin Chem. 2011; 57:490–501.
Article7. Visseren FL, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021; 42:3227–337.8. Yang YS, Kim HL, Kim SH, Moon MK; Committee of Clinical Practice Guideline; Korean Diabetes Association and Clinical Practice Guideline Committee, et al. Lipid management in Korean people with type 2 diabetes mellitus: Korean Diabetes Association and Korean Society of Lipid and Atherosclerosis consensus statement. J Lipid Atheroscler. 2023; 12:12–22.
Article9. Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci U S A. 2005; 102:8132–7.
Article10. Gebel T, Arand M, Oesch F. Induction of the peroxisome proliferator activated receptor by fenofibrate in rat liver. FEBS Lett. 1992; 309:37–40.
Article11. McKeage K, Keating GM. Fenofibrate: a review of its use in dyslipidaemia. Drugs. 2011; 71:1917–46.12. Farnier M, Freeman MW, Macdonell G, Perevozskaya I, Davies MJ, Mitchel YB, et al. Efficacy and safety of the coadministration of ezetimibe with fenofibrate in patients with mixed hyperlipidaemia. Eur Heart J. 2005; 26:897–905.
Article13. McKenney JM, Farnier M, Lo KW, Bays HE, Perevozkaya I, Carlson G, et al. Safety and efficacy of long-term co-administration of fenofibrate and ezetimibe in patients with mixed hyperlipidemia. J Am Coll Cardiol. 2006; 47:1584–7.
Article14. Oikawa S, Yamashita S, Nakaya N, Sasaki J, Kono S; Effect of Fenofibrate and Ezetimibe Combination Treatment on Lipid (EFECTL) Study Investigators. Efficacy and safety of longterm coadministration of fenofibrate and ezetimibe in patients with combined hyperlipidemia: results of the EFECTL study. J Atheroscler Thromb. 2017; 24:77–94.
Article15. Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbalan R, Spinar J, et al. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: results from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2018; 137:1571–82.
Article16. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387–97.
Article17. Kim BK, Hong SJ, Lee YJ, Hong SJ, Yun KH, Hong BK, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022; 400:380–90.
Article18. Lee YJ, Cho JY, You SC, Lee YH, Yun KH, Cho YH, et al. Moderate-intensity statin with ezetimibe vs. high-intensity statin in patients with diabetes and atherosclerotic cardiovascular disease in the RACING trial. Eur Heart J. 2023; 44:972–83.
Article19. Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007; 370:1687–97.
Article20. Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014; 121:2443–51.
Article21. Kim NH, Choi J, Kim YH, Lee H, Kim SG. Addition of fenofibrate to statins is associated with risk reduction of diabetic retinopathy progression in patients with type 2 diabetes and metabolic syndrome: a propensity-matched cohort study. Diabetes Metab. 2023; 49:101428.
Article22. Frazier R, Mehta R, Cai X, Lee J, Napoli S, Craven T, et al. Associations of fenofibrate therapy with incidence and progression of CKD in patients with type 2 diabetes. Kidney Int Rep. 2018; 4:94–102.
Article23. Kähm K, Laxy M, Schneider U, Rogowski WH, Lhachimi SK, Holle R. Health care costs associated with incident complications in patients with type 2 diabetes in Germany. Diabetes Care. 2018; 41:971–8.
Article24. Dal Canto E, Ceriello A, Ryden L, Ferrini M, Hansen TB, Schnell O, et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019; 26(2 suppl):25–32.
Article25. Savelieff MG, Callaghan BC, Feldman EL. The emerging role of dyslipidemia in diabetic microvascular complications. Curr Opin Endocrinol Diabetes Obes. 2020; 27:115–23.
Article26. Wassmer G, Brannath W. Group sequential and confirmatory adaptive designs in clinical trials. Heidelberg: Springer;2016.27. Fang M, Selvin E. Thirty-year trends in complications in U.S. adults with newly diagnosed type 2 diabetes. Diabetes Care. 2021; 44:699–706.
Article28. An J, Nichols GA, Qian L, Munis MA, Harrison TN, Li Z, et al. Prevalence and incidence of microvascular and macrovascular complications over 15 years among patients with incident type 2 diabetes. BMJ Open Diabetes Res Care. 2021; 9:e001847.
Article29. Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, et al. The residual risk reduction initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008; 102(10 Suppl):1K–34K.
Article30. Fox KM, Tai MH, Kostev K, Hatz M, Qian Y, Laufs U. Treatment patterns and low-density lipoprotein cholesterol (LDL-C) goal attainment among patients receiving high- or moderate-intensity statins. Clin Res Cardiol. 2018; 107:380–8.
Article31. Jin ES, Shim JS, Kim SE, Bae JH, Kang S, Won JC, et al. Dyslipidemia fact sheet in South Korea, 2022. Diabetes Metab J. 2023; 47:632–42.
Article32. Yun SJ, Jeong IK, Cha JH, Lee J, Cho HC, Choi SH, et al. Current status of low-density lipoprotein cholesterol target achievement in patients with type 2 diabetes mellitus in Korea compared with recent guidelines. Diabetes Metab J. 2021; 46:464–75.
Article33. Banach M, Stulc T, Dent R, Toth PP. Statin non-adherence and residual cardiovascular risk: there is need for substantial improvement. Int J Cardiol. 2016; 225:184–96.
Article34. Bates TR, Connaughton VM, Watts GF. Non-adherence to statin therapy: a major challenge for preventive cardiology. Expert Opin Pharmacother. 2009; 10:2973–85.
Article35. Wouters H, Van Dijk L, Geers HC, Winters NA, Van Geffen EC, Stiggelbout AM, et al. Understanding statin non-adherence: knowing which perceptions and experiences matter to different patients. PLoS One. 2016; 11:e0146272.
Article36. Bae JH, Jin ES, Kim SE, Kang S, Lee JY, Kim M, et al. Public awareness of dyslipidemia among the Korean population: a survey study. J Lipid Atheroscler. 2023; 12:307–14.
Article37. Gagne C, Bays HE, Weiss SR, Mata P, Quinto K, Melino M, et al. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol. 2002; 90:1084–91.
Article38. Mikhailidis DP, Lawson RW, McCormick AL, Sibbring GC, Tershakovec AM, Davies GM, et al. Comparative efficacy of the addition of ezetimibe to statin vs statin titration in patients with hypercholesterolaemia: systematic review and meta-analysis. Curr Med Res Opin. 2011; 27:1191–210.39. Choi H, Kang SH, Jeong SW, Yoon CH, Youn TJ, Song WH, et al. Lipid-lowering efficacy of combination therapy with moderate-intensity statin and ezetimibe versus high-intensity statin monotherapy: a randomized, open-label, non-inferiority trial from Korea. J Lipid Atheroscler. 2023; 12:277–89.
Article40. Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008; 51:724–30.
Article41. Faergeman O, Holme I, Fayyad R, Bhatia S, Grundy SM, Kastelein JJ, et al. Plasma triglycerides and cardiovascular events in the Treating to New Targets and Incremental Decrease in End-Points through Aggressive Lipid Lowering trials of statins in patients with coronary artery disease. Am J Cardiol. 2009; 104:459–63.
Article42. ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362:1563–74.
Article43. Saely CH, Rein P, Drexel H. Combination lipid therapy in type 2 diabetes. N Engl J Med. 2010; 363:692.
Article44. Kim NH, Han KH, Choi J, Lee J, Kim SG. Use of fenofibrate on cardiovascular outcomes in statin users with metabolic syndrome: propensity matched cohort study. BMJ. 2019; 366:l5125.
Article45. Kim NH, Kim SG. Fibrates revisited: potential role in cardiovascular risk reduction. Diabetes Metab J. 2020; 44:213–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of Moderate-Intensity Statin and Ezetimibe Combination Therapy Versus High-Intensity Statin Monotherapy in Patients With Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- Role of Fenofibrate Use in Dyslipidemia and Related Comorbidities in the Asian Population: A Narrative Review
- What Do We Get from Recent Statin and CETP Inhibitors Trials?
- Lipid-Lowering Efficacy of Combination Therapy With ModerateIntensity Statin and Ezetimibe Versus High-Intensity Statin Monotherapy: A Randomized, Open-Label, NonInferiority Trial From Korea
- Pharmacological Strategies beyond Statins: Ezetimibe and PCSK9 Inhibitors