Endocrinol Metab.
2024 Oct;39(5):686-692. 10.3803/EnM.2024.2068.
Thyroid Hormone-Mediated Selective Autophagy and Its Implications in Countering Metabolic Dysfunction-Associated Steatotic Liver Disease
- Affiliations
-
- 1Department of Endocrinology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
- KMID: 2560275
- DOI: http://doi.org/10.3803/EnM.2024.2068
Abstract
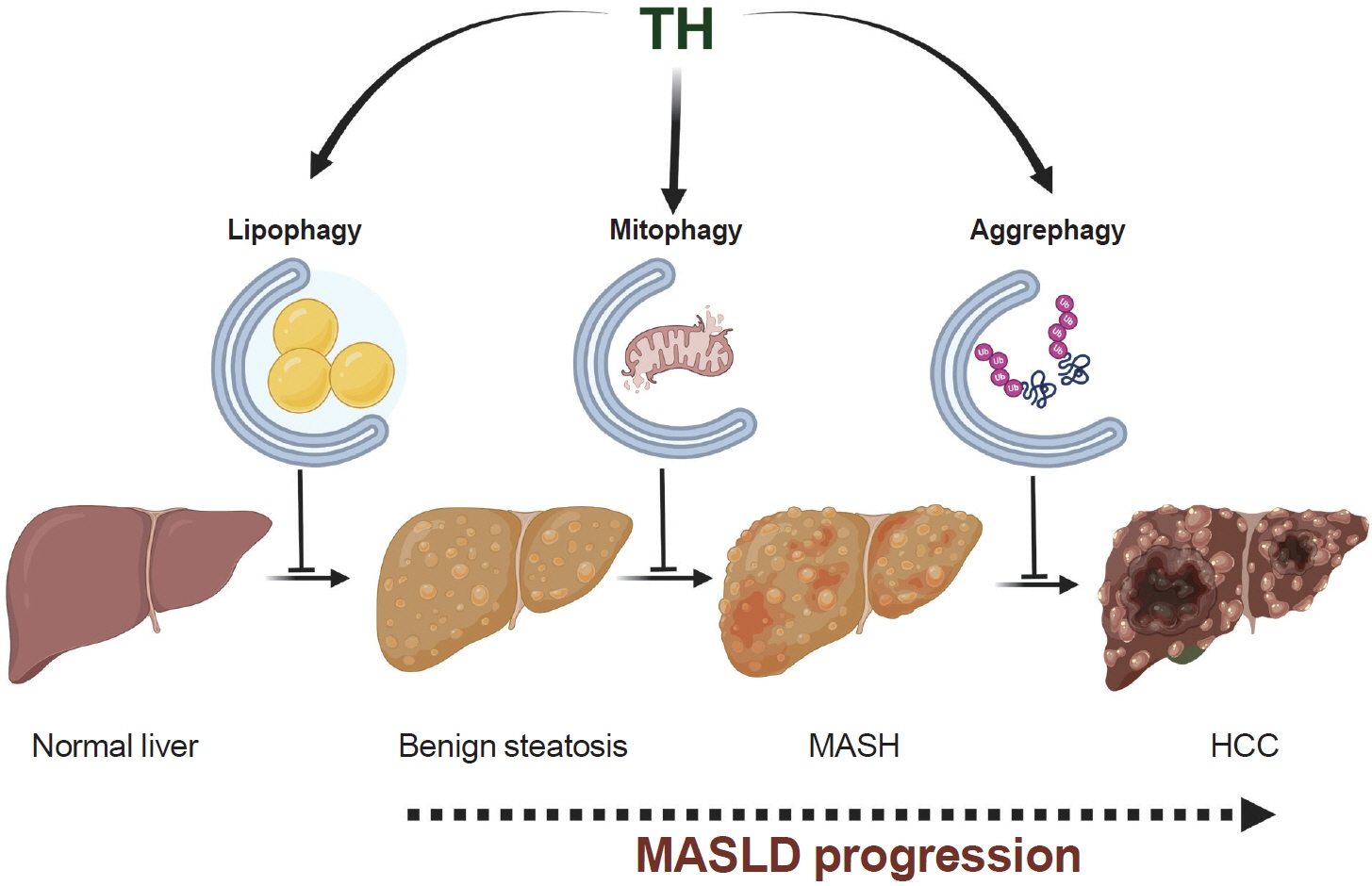
- The influence of thyroid hormone (TH) on liver metabolism has attracted the attention of pharmacologists seeking new treatments for metabolic dysfunction-associated steatotic liver disease (MASLD), an increasingly common metabolic disorder. In this context, the selective induction of autophagy by TH in preclinical models has been identified as a promising mechanism. In this process, TH clears intrahepatic fat through lipophagy while protecting against inflammation and mitochondrial damage in hepatocytes via mitophagy. Furthermore, TH-induced aggrephagy may represent a protective mechanism to mitigate the development of MASLD-associated hepatocellular carcinoma. Considering the defects in autophagy observed during the progression of human MASLD, the induction of autophagy by TH, its metabolites, and its analogs represent a novel strategy to combat hepatic damage across the MASLD spectrum.
Keyword
Figure
Reference
-
1. Sinha RA, Yen PM. Metabolic messengers: thyroid hormones. Nat Metab. 2024; 6:639–50.
Article2. He W, An X, Li L, Shao X, Li Q, Yao Q, et al. Relationship between hypothyroidism and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2017; 8:335.
Article3. Mantovani A, Nascimbeni F, Lonardo A, Zoppini G, Bonora E, Mantzoros CS, et al. Association between primary hypothyroidism and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Thyroid. 2018; 28:1270–84.
Article4. Zeng X, Li B, Zou Y. The relationship between non-alcoholic fatty liver disease and hypothyroidism: a systematic review and meta-analysis. Medicine (Baltimore). 2021; 100:e25738.5. Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic dysfunction-associated steatotic liver disease (MASLD): a state-of-the-art review. J Obes Metab Syndr. 2023; 32:197–213.
Article6. Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. 2024; 35:P697–707.
Article7. Targher G, Byrne CD, Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024; 73:691–702.
Article8. Machado MV. MASLD treatment: a shift in the paradigm is imminent. Front Med (Lausanne). 2023; 10:1316284.9. Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018; 14:259–69.
Article10. Shatz O, Elazar Z. Autophagy in a nutshell. FEBS Lett. 2024; 598:7–8.
Article11. Vargas JN, Hamasaki M, Kawabata T, Youle RJ, Yoshimori T. The mechanisms and roles of selective autophagy in mammals. Nat Rev Mol Cell Biol. 2023; 24:167–85.
Article12. Sinha RA, You SH, Zhou J, Siddique MM, Bay BH, Zhu X, et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J Clin Invest. 2012; 122:2428–38.
Article13. Sinha RA, Singh BK, Zhou J, Wu Y, Farah BL, Ohba K, et al. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy. 2015; 11:1341–57.
Article14. Chi HC, Chen SL, Tsai CY, Chuang WY, Huang YH, Tsai MM, et al. Thyroid hormone suppresses hepatocarcinogenesis via DAPK2 and SQSTM1-dependent selective autophagy. Autophagy. 2016; 12:2271–85.
Article15. Kokkorakis M, Boutari C, Hill MA, Kotsis V, Loomba R, Sanyal AJ, et al. Resmetirom, the first approved drug for the management of metabolic dysfunction-associated steatohepatitis: trials, opportunities, and challenges. Metabolism. 2024; 154:155835.
Article16. Dashti Z, Yousefi Z, Kiani P, Taghizadeh M, Maleki MH, Borji M, et al. Autophagy and the unfolded protein response shape the non-alcoholic fatty liver landscape: decoding the labyrinth. Metabolism. 2024; 154:155811.
Article17. Jin S, Li Y, Xia T, Liu Y, Zhang S, Hu H, et al. Mechanisms and therapeutic implications of selective autophagy in nonalcoholic fatty liver disease. J Adv Res. 2024; Feb. 1. [Epub]. https://doi.org/10.1016/j.jare.2024.01.027.
Article18. Ren Q, Sun Q, Fu J. Dysfunction of autophagy in high-fat diet-induced non-alcoholic fatty liver disease. Autophagy. 2024; 20:221–41.
Article19. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011; 13:132–41.
Article20. Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011; 27:107–32.
Article21. Di Malta C, Cinque L, Settembre C. Transcriptional regulation of autophagy: mechanisms and diseases. Front Cell Dev Biol. 2019; 7:114.
Article22. Singh BK, Sinha RA, Tripathi M, Mendoza A, Ohba K, Sy JA, et al. Thyroid hormone receptor and ERRα coordinately regulate mitochondrial fission, mitophagy, biogenesis, and function. Sci Signal. 2018; 11:eaam5855.
Article23. Zachari M, Ganley IG. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017; 61:585–96.
Article24. Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi J, et al. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009; 284:31484–92.25. Singh BK, Sinha RA, Zhou J, Xie SY, You SH, Gauthier K, et al. FoxO1 deacetylation regulates thyroid hormone-induced transcription of key hepatic gluconeogenic genes. J Biol Chem. 2013; 288:30365–72.
Article26. Singh BK, Sinha RA, Zhou J, Tripathi M, Ohba K, Wang ME, et al. Hepatic FOXO1 target genes are co-regulated by thyroid hormone via RICTOR protein deacetylation and MTORC2-AKT protein inhibition. J Biol Chem. 2016; 291:198–214.
Article27. Napolitano G, Ballabio A. TFEB at a glance. J Cell Sci. 2016; 129:2475–81.
Article28. Iannucci LF, Cioffi F, Senese R, Goglia F, Lanni A, Yen PM, et al. Metabolomic analysis shows differential hepatic effects of T2 and T3 in rats after short-term feeding with high fat diet. Sci Rep. 2017; 7:2023.
Article29. Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009; 458:1131–5.
Article30. Han YH, He XM, Jin MH, Sun HN, Kwon T. Lipophagy: a potential therapeutic target for nonalcoholic and alcoholic fatty liver disease. Biochem Biophys Res Commun. 2023; 672:36–44.
Article31. Tseng YH, Ke PY, Liao CJ, Wu SM, Chi HC, Tsai CY, et al. Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism. Autophagy. 2014; 10:20–31.
Article32. Cang X, Wang Y, Zeng J, Gao J, Yu Q, Lu C, et al. C9orf72 knockdown alleviates hepatic insulin resistance by promoting lipophagy. Biochem Biophys Res Commun. 2022; 588:15–22.
Article33. Yoo J, Jeong IK, Ahn KJ, Chung HY, Hwang YC. Fenofibrate, a PPARα agonist, reduces hepatic fat accumulation through the upregulation of TFEB-mediated lipophagy. Metabolism. 2021; 120:154798.
Article34. Bruinstroop E, Dalan R, Cao Y, Bee YM, Chandran K, Cho LW, et al. Low-dose levothyroxine reduces intrahepatic lipid content in patients with type 2 diabetes mellitus and NAFLD. J Clin Endocrinol Metab. 2018; 103:2698–706.
Article35. Jun DW, Cho WK, Jun JH, Kwon HJ, Jang KS, Kim HJ, et al. Prevention of free fatty acid-induced hepatic lipotoxicity by carnitine via reversal of mitochondrial dysfunction. Liver Int. 2011; 31:1315–24.
Article36. Rosca MG, Vazquez EJ, Chen Q, Kerner J, Kern TS, Hoppel CL. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes. 2012; 61:2074–83.
Article37. Chi HC, Chen SL, Lin SL, Tsai CY, Chuang WY, Lin YH, et al. Thyroid hormone protects hepatocytes from HBx-induced carcinogenesis by enhancing mitochondrial turnover. Oncogene. 2017; 36:5274–84.
Article38. Yu X, Hao M, Liu Y, Ma X, Lin W, Xu Q, et al. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy. Eur J Pharmacol. 2019; 864:172715.
Article39. Zhou J, Tripathi M, Ho JP, Widjaja AA, Shekeran SG, Camat MD, et al. Thyroid hormone decreases hepatic steatosis, inflammation, and fibrosis in a dietary mouse model of nonalcoholic steatohepatitis. Thyroid. 2022; 32:725–38.
Article40. Moore MP, Cunningham RP, Meers GM, Johnson SA, Wheeler AA, Ganga RR, et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatology. 2022; 76:1452–65.
Article41. Undamatla R, Fagunloye OG, Chen J, Edmunds LR, Murali A, Mills A, et al. Reduced mitophagy is an early feature of NAFLD and liver-specific PARKIN knockout hastens the onset of steatosis, inflammation and fibrosis. Sci Rep. 2023; 13:7575.
Article42. Kim NG, Nguyen PP, Dang H, Kumari R, Garcia G, Esquivel CO, et al. Temporal trends in disease presentation and survival of patients with hepatocellular carcinoma: a realworld experience from 1998 to 2015. Cancer. 2018; 124:2588–98.
Article43. Cho CS, Park HW, Ho A, Semple IA, Kim B, Jang I, et al. Lipotoxicity induces hepatic protein inclusions through TANK binding kinase 1-mediated p62/sequestosome 1 phosphorylation. Hepatology. 2018; 68:1331–46.
Article44. Denk H, Stumptner C, Abuja PM, Zatloukal K. Sequestosome 1/p62-related pathways as therapeutic targets in hepatocellular carcinoma. Expert Opin Ther Targets. 2019; 23:393–406.
Article45. Bauer B, Martens S, Ferrari L. Aggrephagy at a glance. J Cell Sci. 2023; 136:jcs260888.
Article46. Sinha RA. Autophagy: a cellular guardian against hepatic lipotoxicity. Genes (Basel). 2023; 14:553.
Article47. Raza S, Rajak S, Singh R, Zhou J, Sinha RA, Goel A. Cell-type specific role of autophagy in the liver and its implications in non-alcoholic fatty liver disease. World J Hepatol. 2023; 15:1272–83.
Article48. Alim Al-Bari A, Ito Y, Thomes PG, Menon MB, GarciaMacia M, Fadel R, et al. Emerging mechanistic insights of selective autophagy in hepatic diseases. Front Pharmacol. 2023; 14:1149809.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ursolic acid: A promising therapeutic agent for metabolic dysfunction-associated steatotic liver disease via inhibition of SPP1-induced Th17 cell differentiation: Editorial on “Ursolic acid targets secreted phosphoprotein 1 to regulate Th17 cells against metabolic dysfunction-associated steatotic liver disease”
- A leap in the dark: Bariatric surgery for treatment of metabolic dysfunction-associated steatotic liver disease related cirrhosis: Editorial on “Bariatric surgery reduces long-term mortality in patients with metabolic dysfunction-associated steatotic liver disease and cirrhosis”
- Prevalence of steatotic liver disease and associated fibrosis in the general population: An epidemiological survey: Letter to the editor on “Epidemiology of metabolic dysfunction-associated steatotic liver disease”
- MAFLD or MASLD: Which better represents the prognosis of the steatotic liver population: Letter to the editor on “Evolutionary changes in metabolic dysfunction-associated steatotic liver disease and risk of hepatocellular carcinoma: A nationwide cohort study”
- The new definition of metabolic dysfunction-associated steatotic liver disease: the role of ultrasound and elastography