Cancer Res Treat.
2024 Oct;56(4):1240-1251. 10.4143/crt.2023.977.
Clinical Outcomes of Surgery after Neoadjuvant Chemotherapy in Locally Advanced Pancreatic Ductal Adenocarcinoma
- Affiliations
-
- 1Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Brain Korea 21 Project, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2560257
- DOI: http://doi.org/10.4143/crt.2023.977
Abstract
- Purpose
Clinical outcomes of surgery after neoadjuvant chemotherapy have not been investigated for locally advanced pancreatic cancer (LAPC), despite well-established outcomes in borderline resectable pancreatic cancer (BRPC). This study aimed to investigate the clinical outcomes of patients with LAPC who underwent curative resection following neoadjuvant chemotherapy.
Materials and Methods
We retrospectively reviewed the records of patients diagnosed with pancreatic adenocarcinoma between January 2017 and December 2020.
Results
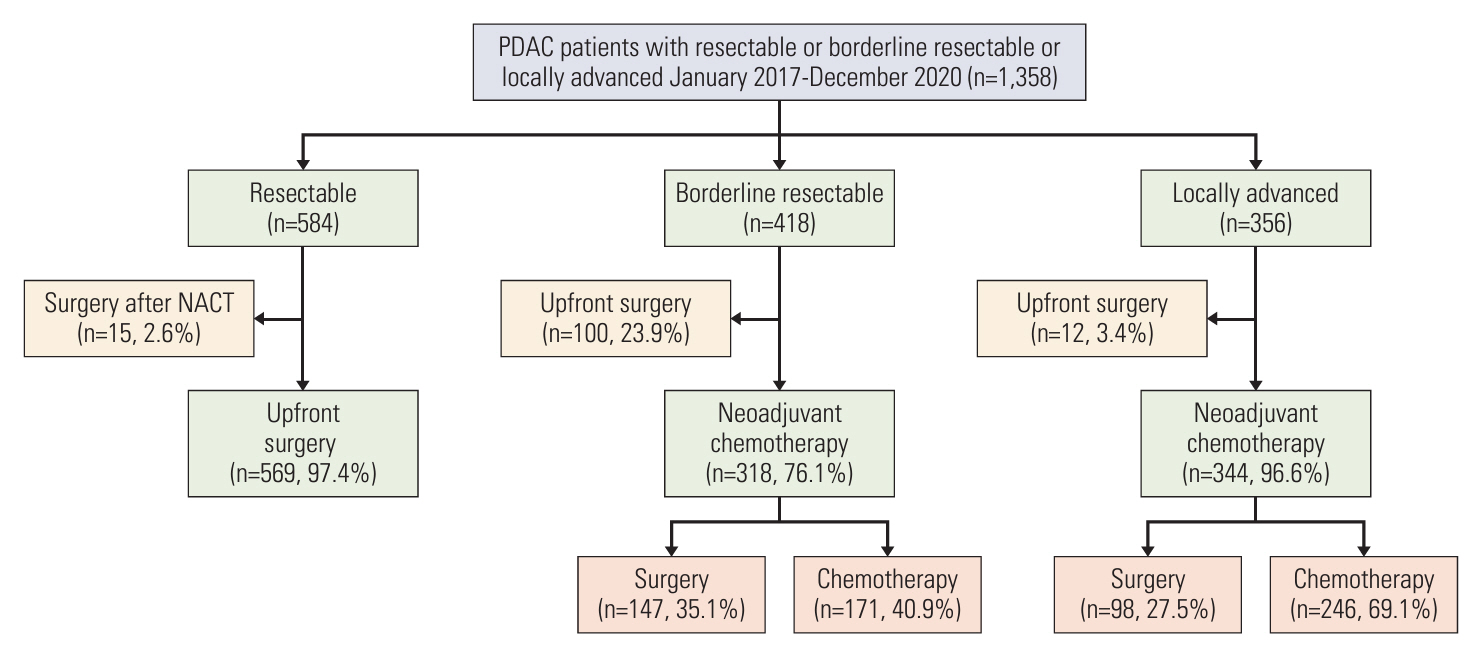
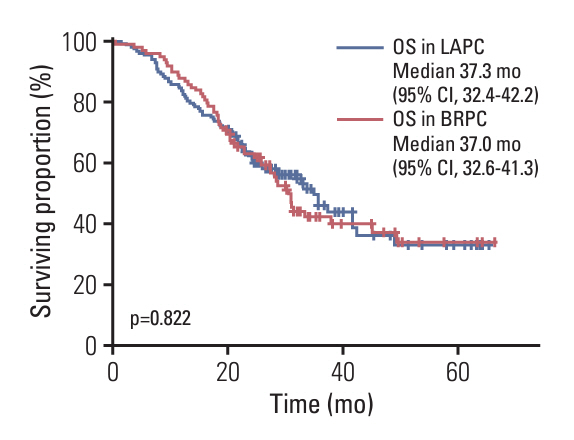
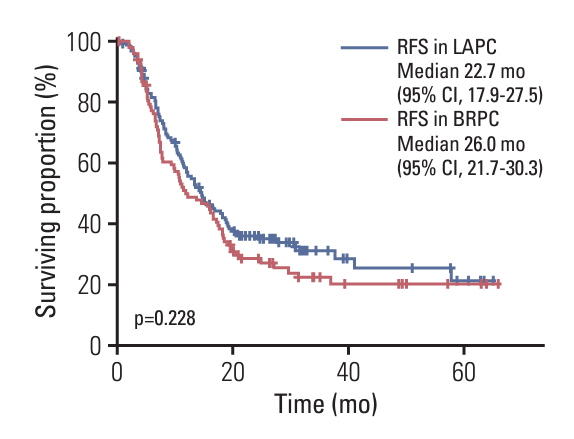
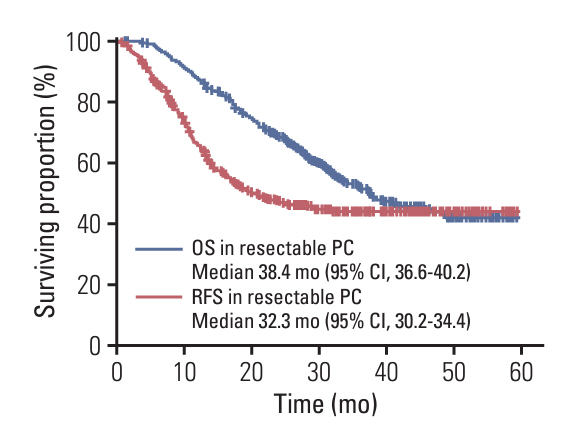
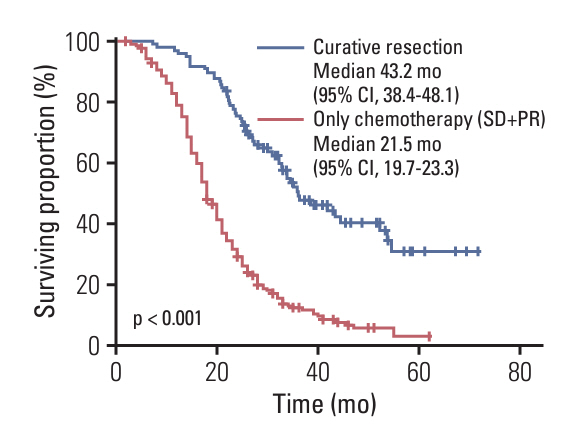
Among 1,358 patients, 260 underwent surgery following neoadjuvant chemotherapy. Among 356 LAPC patients, 98 (27.5%) and 147 (35.1%) of 418 BRPC patients underwent surgery after neoadjuvant chemotherapy. Compared to resectable pancreatic cancer (resectable PC) with upfront surgery, both LAPC and BRPC exhibited higher rates of venous resection (28.6% vs. 49.0% vs. 4.0%), arterial resection (30.6% vs. 6.8% vs. 0.5%) and greater estimated blood loss (260.5 vs. 213.1 vs. 70.4 mL). However, hospital stay, readmission rates, and postoperative pancreatic fistula rates (grade B or C) did not differ significantly between LAPC, BRPC, and resectable PC. Overall and relapse-free survival did not differ significantly between LAPC and BRPC patients. The median overall survival was 37.3 months for LAPC and 37.0 months for BRPC. The median relapse-free survival was 22.7 months for LAPC and 26.0 months for BRPC.
Conclusion
Overall survival time and postoperative complications in LAPC patients who underwent curative resection following neoadjuvant chemotherapy showed similar results to those of BRPC patients. Further research is needed to identify specific sub-populations of LAPC patients who benefit most from conversion surgery and to minimize postoperative complications.
Keyword
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article2. Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020; 159:335–49.
Article3. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018; 24:4846–61.
Article4. Mohammed S, Van Buren G 2nd, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol. 2014; 20:9354–60.5. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021; 71:7–33.
Article6. Park W, Chawla A, O’Reilly EM. Pancreatic cancer: a review. JAMA. 2021; 326:851–62.7. Ansari D, Tingstedt B, Andersson B, Holmquist F, Sturesson C, Williamsson C, et al. Pancreatic cancer: yesterday, today and tomorrow. Future Oncol. 2016; 12:1929–46.
Article8. Chu LC, Goggins MG, Fishman EK. Diagnosis and detection of pancreatic cancer. Cancer J. 2017; 23:333–42.
Article9. Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009; 16:1727–33.
Article10. Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021; 19:439–57.11. Satoi S, Yamamoto T, Yamaki S, Sakaguchi T, Sekimoto M. Surgical indication for and desirable outcomes of conversion surgery in patients with initially unresectable pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2020; 4:6–13.
Article12. Fukuchi M, Ishiguro T, Ogata K, Suzuki O, Kumagai Y, Ishibashi K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015; 22:3618–24.
Article13. Yoshitomi H, Takano S, Furukawa K, Takayashiki T, Kuboki S, Ohtsuka M. Conversion surgery for initially unresectable pancreatic cancer: current status and unresolved issues. Surg Today. 2019; 49:894–906.
Article14. Amano R, Kimura K, Nakata B, Yamazoe S, Motomura H, Yamamoto A, et al. Pancreatectomy with major arterial resection after neoadjuvant chemoradiotherapy gemcitabine and S-1 and concurrent radiotherapy for locally advanced unresectable pancreatic cancer. Surgery. 2015; 158:191–200.
Article15. Nitsche U, Wenzel P, Siveke JT, Braren R, Holzapfel K, Schlitter AM, et al. Resectability after first-line FOLFIRINOX in initially unresectable locally advanced pancreatic cancer: a single-center experience. Ann Surg Oncol. 2015; 22 Suppl 3:S1212–20.
Article16. Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, et al. Locally advanced pancreatic cancer: neoadjuvant therapy with folfirinox results in resectability in 60% of the patients. Ann Surg. 2016; 264:457–63.17. Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J Clin Oncol. 2022; 40:1220–30.
Article18. Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364:1817–25.
Article19. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013; 369:1691–703.
Article20. Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas. 2015; 44:515–21.21. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article22. Clavien PA, Strasberg SM. Severity grading of surgical complications. Ann Surg. 2009; 250:197–8.
Article23. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017; 161:584–91.24. McCullough TC, Roth JV, Ginsberg PC, Harkaway RC. Estimated blood loss underestimates calculated blood loss during radical retropubic prostatectomy. Urol Int. 2004; 72:13–6.
Article25. Vidri RJ, Olsen WT, Clark DE, Fitzgerald TL. Upfront resection versus neoadjuvant therapy for T1/T2 pancreatic cancer. HPB (Oxford). 2021; 23:279–89.
Article26. Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 2021; 273:341–9.
Article27. Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014; 155:977–88.
Article28. Belfiori G, Fiorentini G, Tamburrino D, Partelli S, Pagnanelli M, Gasparini G, et al. Vascular resection during pancreatectomy for pancreatic head cancer: a technical issue or a prognostic sign? Surgery. 2021; 169:403–10.
Article29. Pecorelli N, Pagnanelli M, Cinelli L, Di Salvo F, Partelli S, Crippa S, et al. Postoperative outcomes and functional recovery after preoperative combination Cchemotherapy for pancreatic cancer: a propensity score-matched study. Front Oncol. 2019; 9:1299.30. Griffin JF, Smalley SR, Jewell W, Paradelo JC, Reymond RD, Hassanein RE, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990; 66:56–61.
Article31. Ferrone CR, Kattan MW, Tomlinson JS, Thayer SP, Brennan MF, Warshaw AL. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol. 2005; 23:7529–35.
Article32. Bratlie SO, Wennerblom J, Vilhav C, Persson J, Rangelova E. Resectable, borderline, and locally advanced pancreatic cancer-“the good, the bad, and the ugly” candidates for surgery? J Gastrointest Oncol. 2021; 12:2450–60.
Article33. Saito T, Ishido K, Kudo D, Kimura N, Wakiya T, Nakayama Y, et al. Combination therapy with gemcitabine and nab-paclitaxel for locally advanced unresectable pancreatic cancer. Mol Clin Oncol. 2017; 6:963–7.
Article34. Satoi S, Yamaue H, Kato K, Takahashi S, Hirono S, Takeda S, et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2013; 20:590–600.
Article35. Wang M, Zhu P, Chen Z, Yang L. Conversion therapy, palliative chemotherapy and surgery, which of these is the best treatment for locally advanced and advanced pancreatic cancer? Anticancer Drugs. 2022; 33:e686–91.
Article36. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997; 15:2403–13.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long Term Complete Response of Unresectable Locally Advanced Pancreatic Cancer after CCRT and Gemcitabine Chemotherapy
- Neoadjuvant and Adjuvant Treatments for Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma: The Current Status of Pancreatic Ductal Adenocarcinoma Treatment in Japan
- Radiologic Evaluation for Resectability of Pancreatic Adenocarcinoma
- Contemporary management of borderline resectable pancreatic ductal adenocarcinoma
- Neoadjuvant Chemotherapy in Asian Patients With Locally Advanced Gastric Cancer