Cancer Res Treat.
2024 Oct;56(4):1126-1135. 10.4143/crt.2024.328.
Identification of New Pathogenic Variants of Hereditary Diffuse Gastric Cancer
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Critical Care Medicine, Seoul National University Hospital, Seoul, Korea
- 3Genomic Medicine Institute, Medical Research Center, Seoul National University, Seoul, Korea
- 4Ewha Biomedical Research Institute, Ewha Womans University Medical Center, Seoul, Korea
- 5Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea
- 6Department of Biochemistry and Molecular Biology, Seoul National University College of Medicine, Seoul, Korea
- 7Cancer Research Institute, Seoul National University, Seoul, Korea
- 8Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
- 9Department of Surgery, Seoul National University Hospital, Seoul, Korea
- KMID: 2560247
- DOI: http://doi.org/10.4143/crt.2024.328
Abstract
- Purpose
Hereditary diffuse gastric cancer (HDGC) presents a significant genetic predisposition, notably linked to mutations in the CDH1 and CTNNA1. However, the genetic basis for over half of HDGC cases remains unidentified. The aim of this study is to identify novel pathogenic variants in HDGC and evaluate their protein expression.
Materials and Methods
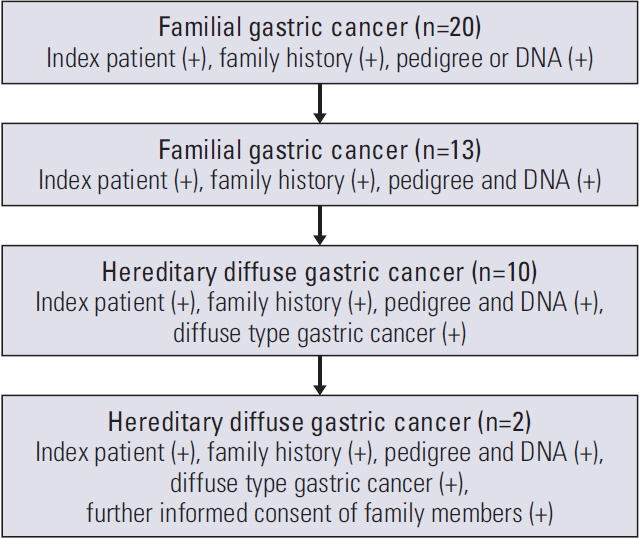
Among 20 qualifying families, two were selected based on available pedigree and DNA. Whole genome sequencing (WGS) on DNA extracted from blood and whole exome sequencing on DNA from formalin-fixed paraffin-embedded tissues were performed to find potential pathogenic variants in HDGC. After selection of a candidate variant, functional validation, and enrichment analysis were performed.
Results
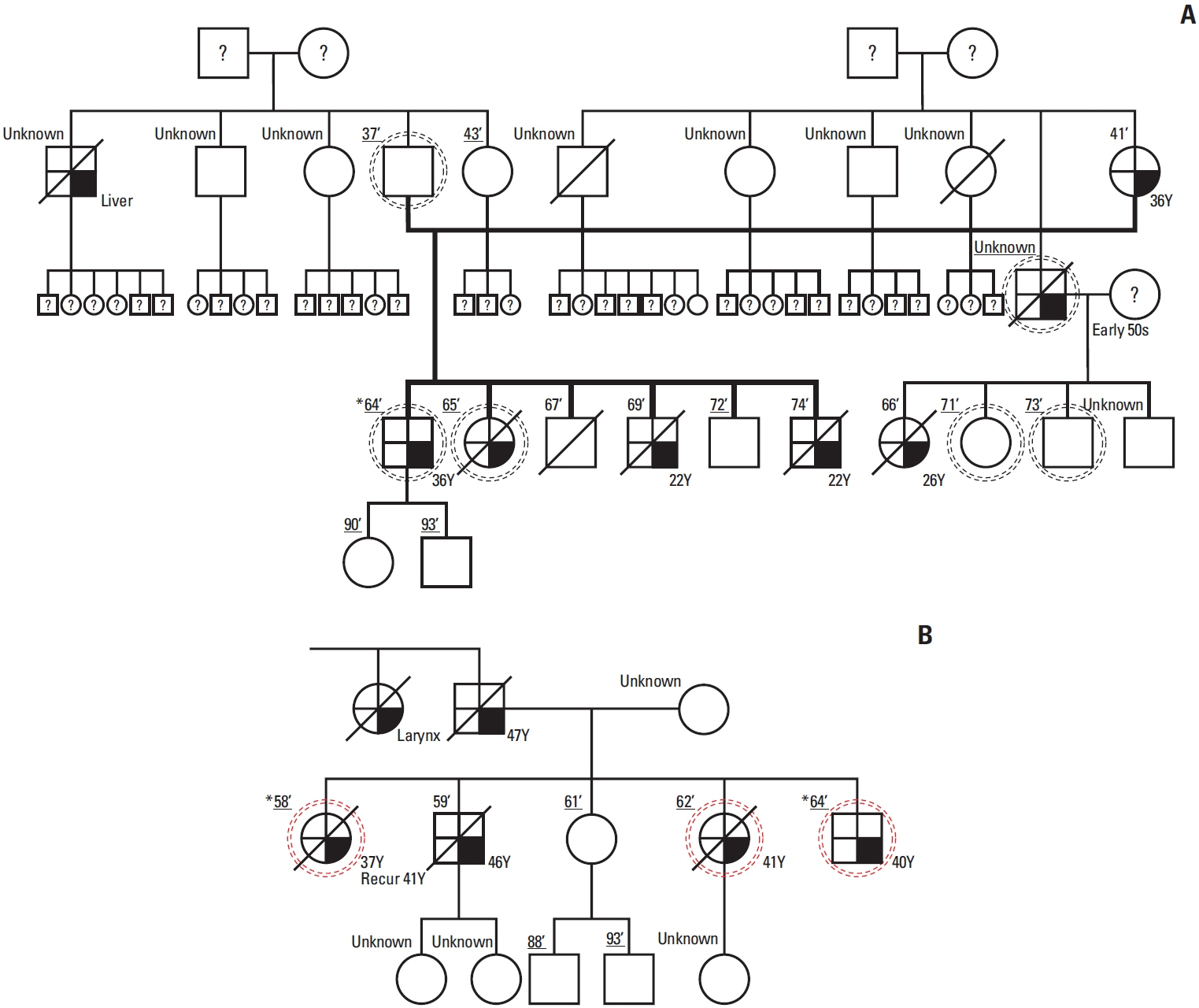
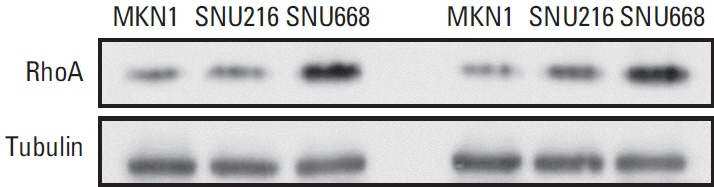
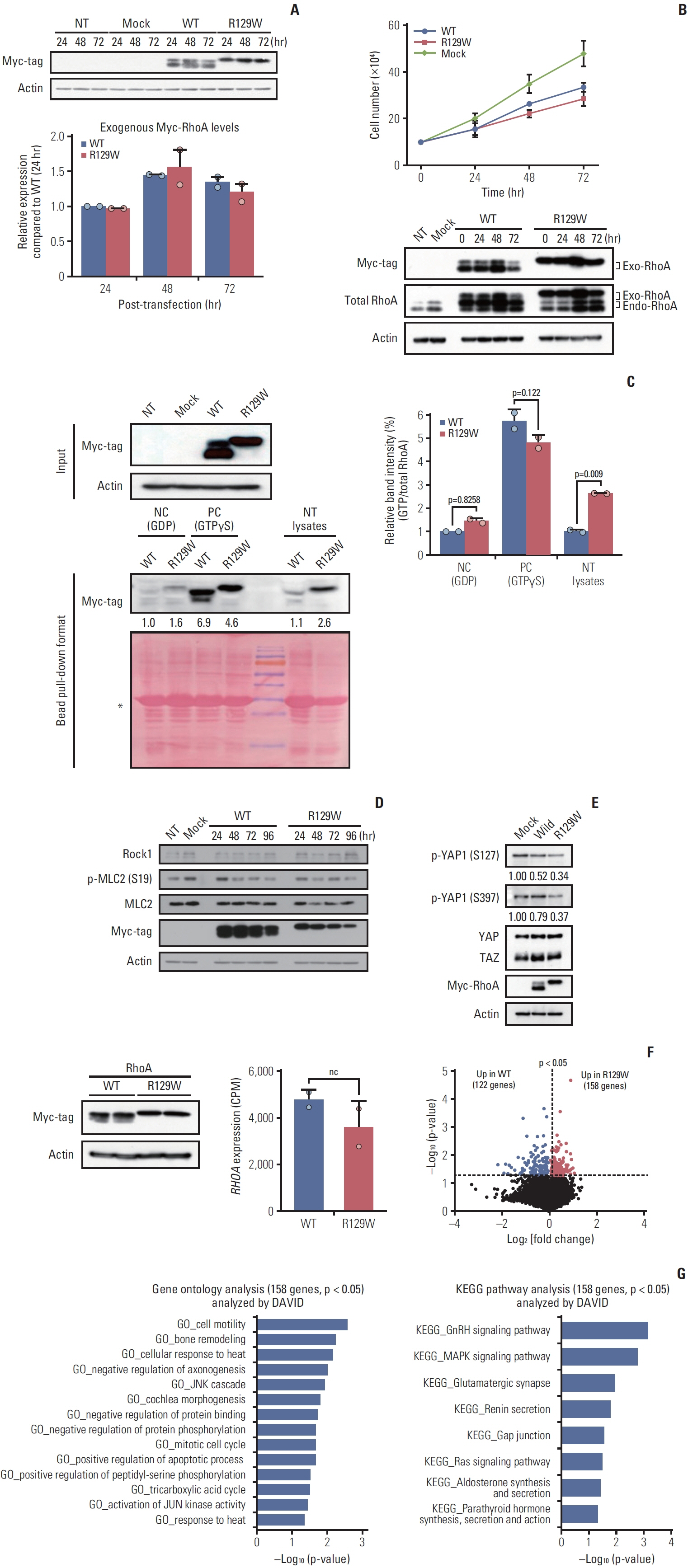
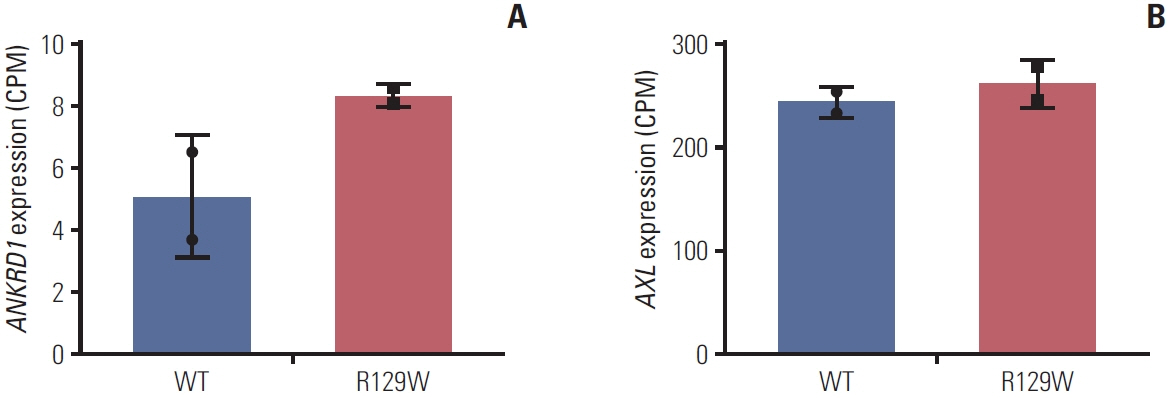
As a result of WGS, three candidate germline mutations (EPHA5, MCOA2, and RHOA) were identified in one family. After literature review and in-silico analyses, the RHOA mutation (R129W) was selected as a candidate. This mutation was found in two gastric cancer patients within the family. In functional validation, it showed RhoA overexpression and a higher GTP-bound state in the RhoaR129W mutant. Decreased phosphorylation at Ser127/397 suggested altered YAP1 regulation in the Rho-ROCK pathway. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses linked RhoaR129W overexpression to changed migration/adhesion in MKN1 cell line. However, this RHOA mutation (R129W) was not found in index patients in other families.
Conclusion
The RHOA mutation (R129W) emerges as a potential causative gene for HDGC, but only in one family, indicating a need for further studies to understand its role in HDGC pathogenesis fully.
Keyword
Figure
Reference
-
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article2. Moller H, Heseltine E, Vainio H. Working group report on schistosomes, liver flukes and Helicobacter pylori. Int J Cancer. 1995; 60:587–9.3. Neugut AI, Hayek M, Howe G. Epidemiology of gastric cancer. Semin Oncol. 1996; 23:281–91.4. Genta RM. Acid suppression and gastric atrophy: sifting fact from fiction. Gut. 1998; 43 Suppl 1:S35–8.
Article5. World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: World Cancer Research Fund, American Institute for Cancer Research;2007.6. Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008; 19:689–701.
Article7. Matsukura N, Onda M, Tokunaga A, Yoshiyuki T, Shimizu Y, Nishi K, et al. Simultaneous gastric cancer in monozygotic twins. Cancer. 1988; 62:2430–5.
Article8. Kinugasa S, Matsuura H, Abe S, Yoshimura H, Tachibana M, Nakamura T, et al. Simultaneous early gastric cancer in identical twins: report of a case. Surg Today. 1996; 26:189–91.
Article9. Shah SC, McKinley M, Gupta S, Peek RM Jr, Martinez ME, Gomez SL. Population-based analysis of differences in gastric cancer incidence among races and ethnicities in individuals age 50 years and older. Gastroenterology. 2020; 159:1705–14.
Article10. van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015; 52:361–74.
Article11. Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998; 392:402–5.
Article12. Grunwald GB. The structural and functional analysis of cadherin calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1993; 5:797–805.
Article13. Shiozaki H, Oka H, Inoue M, Tamura S, Monden M. E-cadherin mediated adhesion system in cancer cells. Cancer. 1996; 77:1605–13.
Article14. Majewski IJ, Kluijt I, Cats A, Scerri TS, de Jong D, Kluin RJ, et al. An alpha-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J Pathol. 2013; 229:621–9.15. Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995; 92:8813–7.
Article16. Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. alphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006; 311:1609–12.
Article17. Jose L. Orgaz VS-M: RhoA. In: Choi S, editor. Encyclopedia of signaling molecules. Cham: Springer; 2018. p. 4681-91.18. Oliveira C, Pinheiro H, Figueiredo J, Seruca R, Carneiro F. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015; 16:e60–70.
Article19. Luo X, Maciaszek JL, Thompson BA, Leong HS, Dixon K, Sousa S, et al. Optimising clinical care through CDH1-specific germline variant curation: improvement of clinical assertions and updated curation guidelines. J Med Genet. 2023; 60:568–75.
Article20. de Assumpcao PB, de Assumpcao PP, Moreira FC, Ribeiro-Dos-Santos A, Vidal AF, Magalhaes L, et al. Incidence of hereditary gastric cancer may be much higher than reported. Cancers (Basel). 2022; 14:6125.
Article21. Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken). 2010; 67:545–54.
Article22. Sadok A, Marshall CJ. Rho GTPases: masters of cell migration. Small GTPases. 2014; 5:e29710.23. Shi J, Wu X, Surma M, Vemula S, Zhang L, Yang Y, et al. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis. 2013; 4:e483.
Article24. Li Y, Wang J, Zhong W. Regulation and mechanism of YAP/TAZ in the mechanical microenvironment of stem cells (Review). Mol Med Rep. 2021; 24:506.25. Almodovar-Garcia K, Kwon M, Samaras SE, Davidson JM. ANKRD1 acts as a transcriptional repressor of MMP13 via the AP-1 site. Mol Cell Biol. 2014; 34:1500–11.26. Samaras SE, Almodovar-Garcia K, Wu N, Yu F, Davidson JM. Global deletion of Ankrd1 results in a wound-healing phenotype associated with dermal fibroblast dysfunction. Am J Pathol. 2015; 185:96–109.
Article27. Jimenez AP, Traum A, Boettger T, Hackstein H, Richter AM, Dammann RH. The tumor suppressor RASSF1A induces the YAP1 target gene ANKRD1 that is epigenetically inactivated in human cancers and inhibits tumor growth. Oncotarget. 2017; 8:88437–52.28. Saab S, Chang OS, Nagaoka K, Hung MC, Yamaguchi H. The potential role of YAP in Axl-mediated resistance to EGFR tyrosine kinase inhibitors. Am J Cancer Res. 2019; 9:2719–29.29. Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST, Chen J, et al. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011; 30:1229–40.
Article30. He L, Lei Y, Hou J, Wu J, Lv G. Implications of the receptor tyrosine kinase Axl in gastric cancer progression. Onco Targets Ther. 2020; 13:5901–11.31. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–74.
Article32. Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014; 46:583–7.
Article33. Nam S, Kim JH, Lee DH. RHOA in gastric cancer: functional roles and therapeutic potential. Front Genet. 2019; 10:438.
Article34. Dvorsky R, Blumenstein L, Vetter IR, Ahmadian MR. Structural insights into the interaction of ROCKI with the switch regions of RhoA. J Biol Chem. 2004; 279:7098–104.
Article35. Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, et al. Wholegenome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014; 46:573–82.
Article36. Cho SY, Park JW, Liu Y, Park YS, Kim JH, Yang H, et al. Sporadic early-onset diffuse gastric cancers have high frequency of somatic CDH1 alterations, but low frequency of somatic RHOA mutations compared with late-onset cancers. Gastroenterology. 2017; 153:536–49.
Article37. Manso R, Sanchez-Beato M, Monsalvo S, Gomez S, Cereceda L, Llamas P, et al. The RHOA G17V gene mutation occurs frequently in peripheral T-cell lymphoma and is associated with a characteristic molecular signature. Blood. 2014; 123:2893–4.
Article38. Chang HR, Nam S, Lee J, Kim JH, Jung HR, Park HS, et al. Systematic approach identifies RHOA as a potential biomarker therapeutic target for Asian gastric cancer. Oncotarget. 2016; 7:81435–51.
Article39. Yoon C, Cho SJ, Aksoy BA, Park DJ, Schultz N, Ryeom SW, et al. Chemotherapy resistance in diffuse-type gastric adenocarcinoma is mediated by RhoA activation in cancer stem-like cells. Clin Cancer Res. 2016; 22:971–83.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Validity of Next-Generation Sequencing Multi-Gene Panel Testing for Detecting Pathogenic Variants in Patients With Hereditary Breast-Ovarian Cancer Syndrome
- Applying Functional Assay Evidence to Interpret Sequence Variants Identified in Hereditary Cancer Genes
- Utility of Next-Generation Sequencing Panel Including Hereditary Breast and Ovarian Cancer-Related Genes for Pathogenic Variant Detection
- Diagnostic Effectiveness of Copy Number Variation Detection Using Multiplex Ligation-Dependent Probe Amplification in Patients with Lynch Syndrome-Related Cancer
- BRCA1/BRCA2 Pathogenic Variant Breast Cancer: Treatment and Prevention Strategies