Cancer Res Treat.
2024 Oct;56(4):1084-1095. 10.4143/crt.2024.084.
Adjuvant Pembrolizumab in Patients with Stage IIIA/N2 Non–Small Cell Lung Cancer Completely Resected after Neoadjuvant Concurrent Chemoradiation: A Prospective, Open-Label, Single-Arm, Phase 2 Trial
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Radiation Oncology, Yonsei Cancer Center, Heavy Ion Therapy Research Institute, Yonsei University College of Medicine, Seoul, Korea
- 3Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea
- 4Department of Thoracic and Cardiovascular Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2560243
- DOI: http://doi.org/10.4143/crt.2024.084
Abstract
- Purpose
Optimal treatment for stage IIIA/N2 non–small cell lung cancer (NSCLC) is controversial. We aimed to assess the efficacy and safety of adjuvant pembrolizumab for stage IIIA/N2 NSCLC completely resected after neoadjuvant concurrent chemoradiation therapy (CCRT).
Materials and Methods
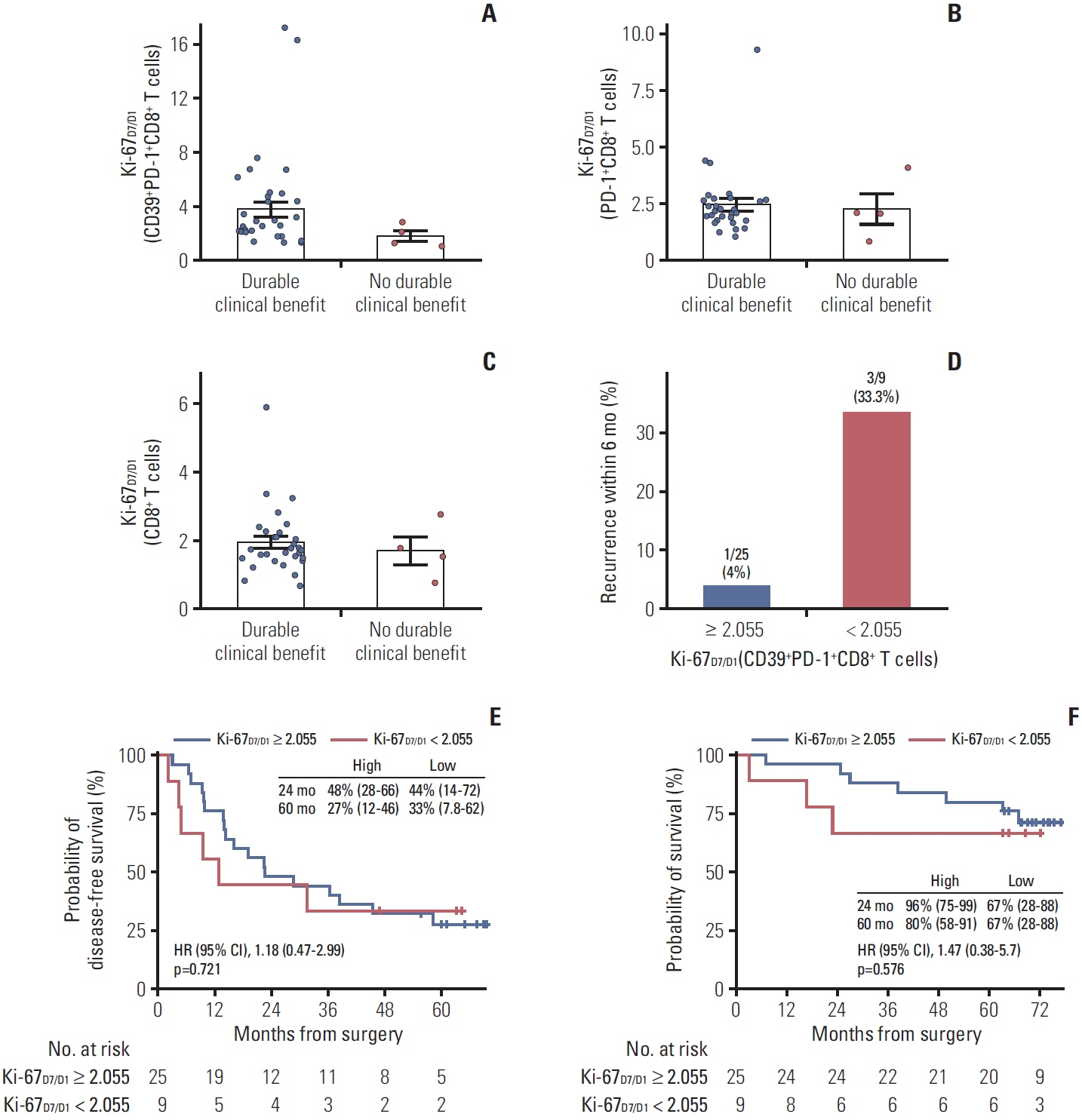
In this open-label, single-center, single-arm phase 2 trial, patients with stage IIIA/N2 NSCLC received adjuvant pembrolizumab for up to 2 years after complete resection following neoadjuvant CCRT. The primary endpoint was disease-free survival (DFS). Secondary endpoints included overall survival (OS) and safety. As an exploratory biomarker analysis, we evaluated the proliferative response of blood CD39+PD-1+CD8+ T cells using fold changes in the percentage of proliferating Ki-67+ cells from days 1 to 7 of cycle 1 (Ki-67D7/D1).
Results
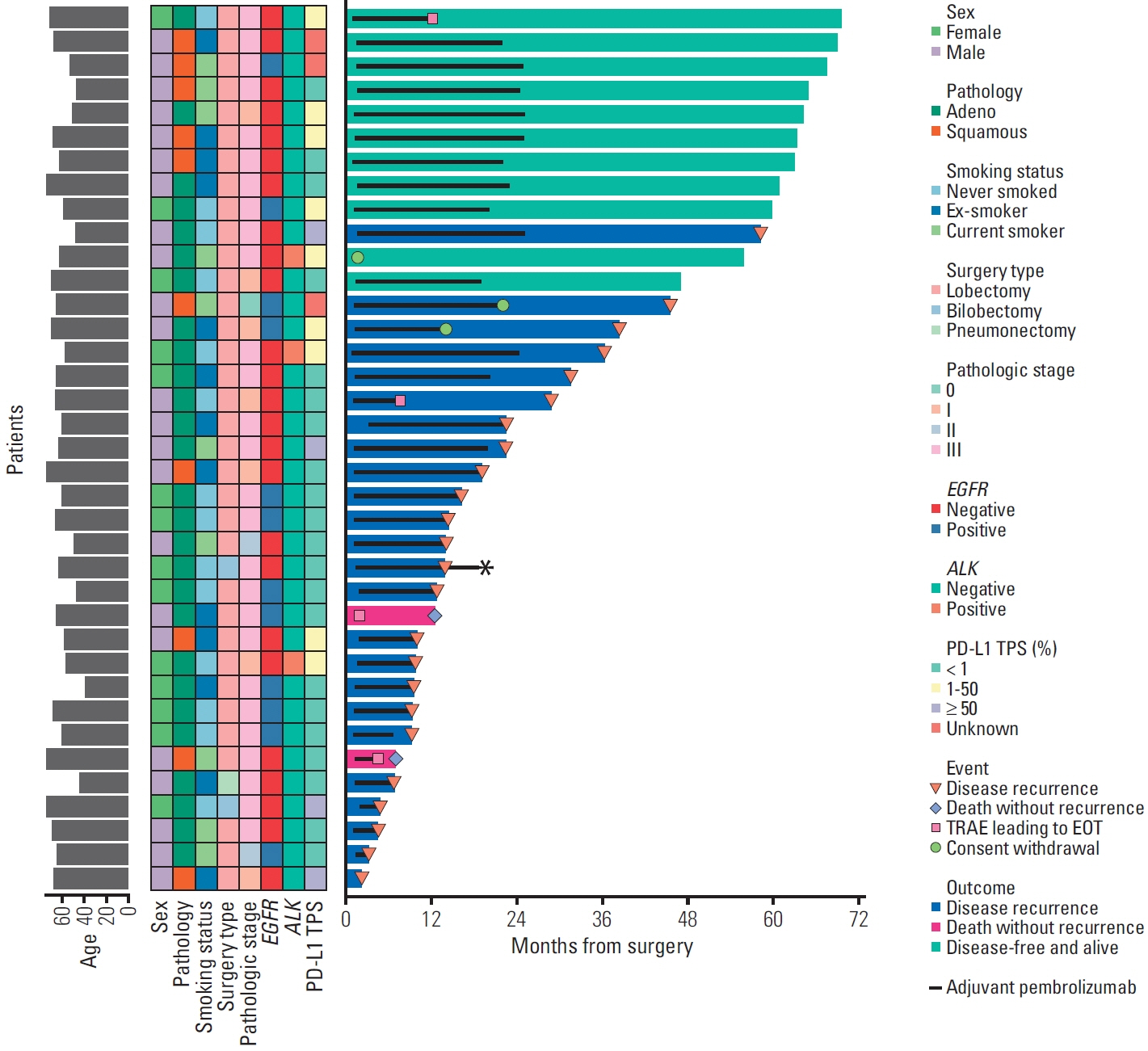
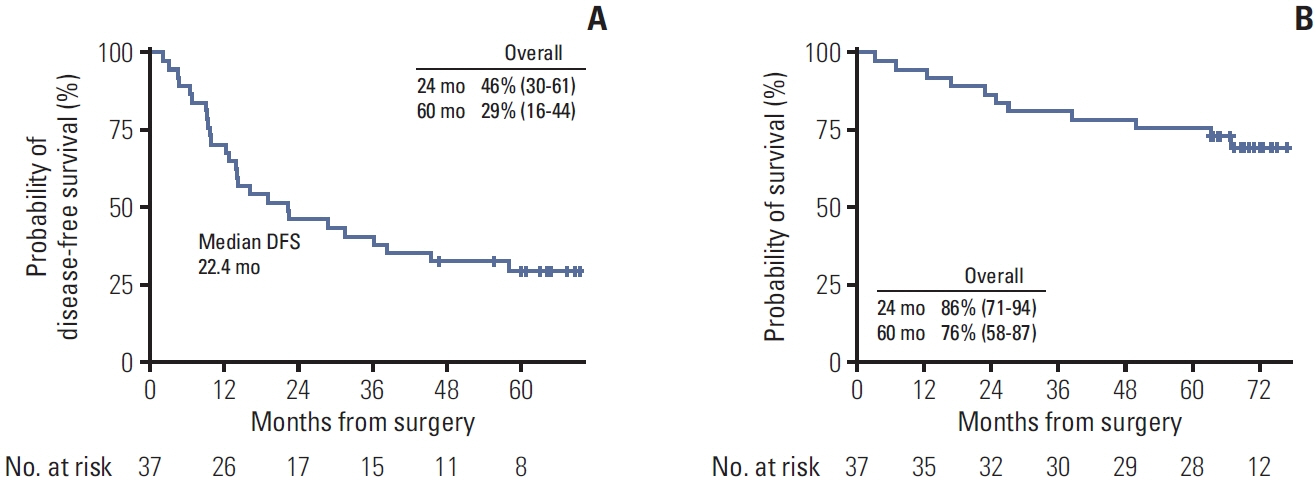
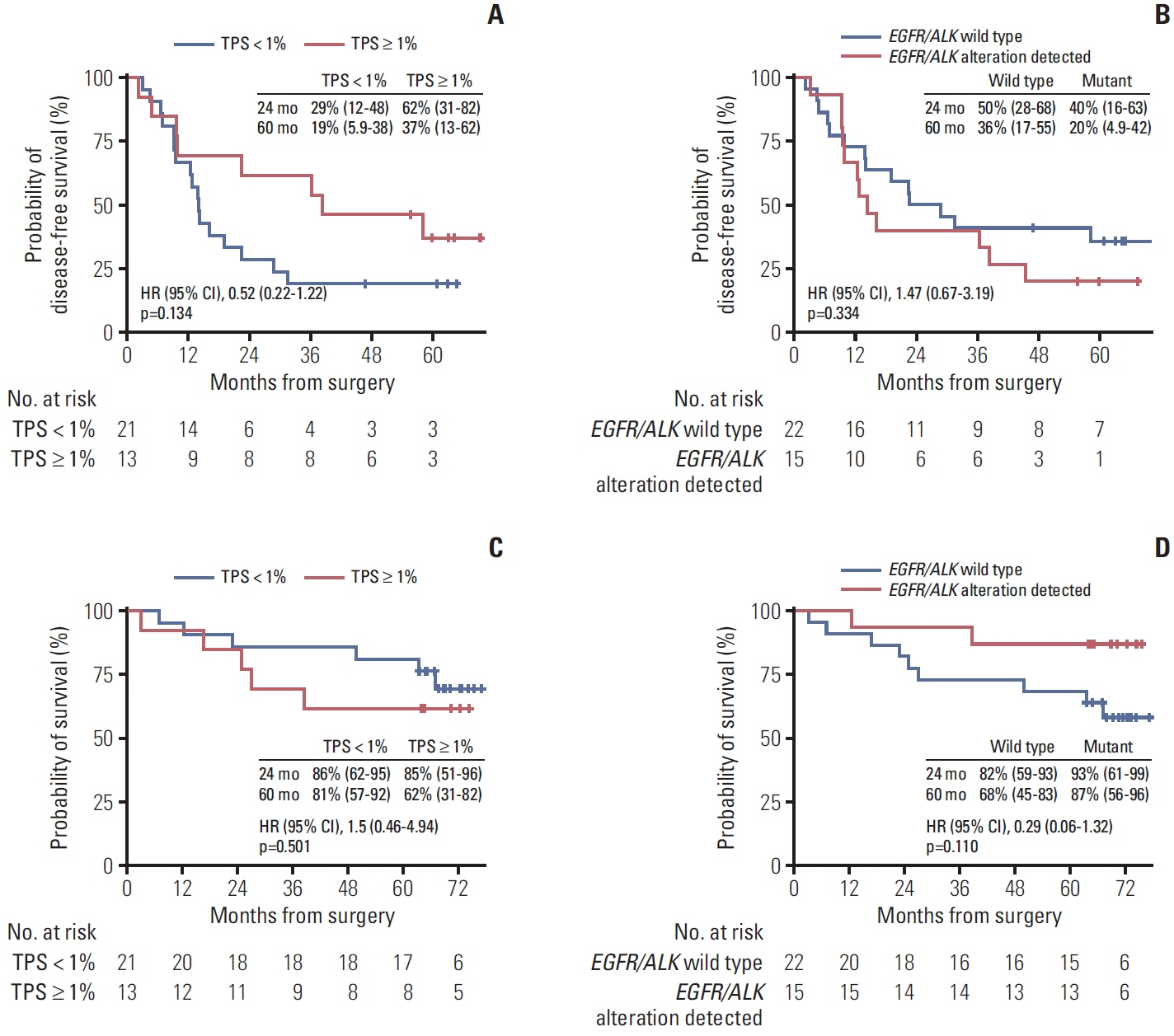
Between October 2017 and October 2018, 37 patients were enrolled. Twelve (32%) and three (8%) patients harbored EGFR and ALK alterations, respectively. Of 34 patients with programmed cell death ligand 1 assessment, 21 (62%), nine (26%), and four (12%) had a tumor proportion score of < 1%, 1%-50%, and ≥ 50%, respectively. The median follow-up was 71 months. The median DFS was 22.4 months in the overall population, with a 5-year DFS rate of 29%. The OS rate was 86% at 2 years and 76% at 5 years. Patients with tumor recurrence within 6 months had a significantly lower Ki-67D7/D1 among CD39+PD-1+CD8+ T cells than those without (p=0.036). No new safety signals were identified.
Conclusion
Adjuvant pembrolizumab may offer durable disease control in a subset of stage IIIA/N2 NSCLC patients after neoadjuvant CCRT and surgery.
Figure
Reference
-
References
1. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. Cham: Springer;2017.2. NSCLC Meta-analyses Collaborative Group, Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010; 375:1267–77.
Article3. NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014; 383:1561–71.4. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016; 11:39–51.5. Chen Y, Peng X, Zhou Y, Xia K, Zhuang W. Comparing the benefits of chemoradiotherapy and chemotherapy for resectable stage III A/N2 non-small cell lung cancer: a meta-analysis. World J Surg Oncol. 2018; 16:8.
Article6. Albain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009; 374:379–86.
Article7. Kim HK, Cho JH, Choi YS, Zo JI, Shim YM, Park K, et al. Outcomes of neoadjuvant concurrent chemoradiotherapy followed by surgery for non-small-cell lung cancer with N2 disease. Lung Cancer. 2016; 96:56–62.
Article8. Daly ME, Singh N, Ismaila N, Antonoff MB, Arenberg DA, Bradley J, et al. Management of stage III non-small-cell lung cancer: ASCO guideline. J Clin Oncol. 2022; 40:1356–84.
Article9. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022; 386:1973–85.
Article10. Felip E, Altorki N, Zhou C, Csoszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021; 398:1344–57.
Article11. O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022; 23:1274–86.
Article12. Provencio M, Nadal E, Insa A, Garcia-Campelo MR, CasalRubio J, Domine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020; 21:1413–22.
Article13. Provencio M, Nadal E, Gonzalez-Larriba JL, Martinez-Marti A, Bernabe R, Bosch-Barrera J, et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2023; 389:504–13.
Article14. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al. Perioperative pembrolizumab for early-stage non-smallcell lung cancer. N Engl J Med. 2023; 389:491–503.
Article15. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023; 389:1672–84.
Article16. Singh N, Daly ME, Ismaila N; Management of Stage III NSCLC Guideline Expert Panel. Management of stage III non-small-cell lung cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2023; 41:4430–2.
Article17. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019; 30:1321–8.
Article18. Katakami N, Tada H, Mitsudomi T, Kudoh S, Senba H, Matsui K, et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer. 2012; 118:6126–35.
Article19. Pless M, Stupp R, Ris HB, Stahel RA, Weder W, Thierstein S, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015; 386:1049–56.
Article20. Mouillet G, Monnet E, Milleron B, Puyraveau M, Quoix E, David P, et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol. 2012; 7:841–9.
Article21. Scagliotti GV, Pastorino U, Vansteenkiste JF, Spaggiari L, Facciolo F, Orlowski TM, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol. 2012; 30:172–8.
Article22. Martin J, Ginsberg RJ, Venkatraman ES, Bains MS, Downey RJ, Korst RJ, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol. 2002; 20:1989–95.
Article23. Betticher DC, Hsu Schmitz SF, Totsch M, Hansen E, Joss C, von Briel C, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol. 2003; 21:1752–9.
Article24. Thomas M, Rube C, Hoffknecht P, Macha HN, Freitag L, Linder A, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008; 9:636–48.
Article25. Saw SP, Ong BH, Chua KL, Takano A, Tan DS. Revisiting neoadjuvant therapy in non-small-cell lung cancer. Lancet Oncol. 2021; 22:e501–16.
Article26. Kim KH, Cho J, Ku BM, Koh J, Sun JM, Lee SH, et al. The first-week proliferative response of peripheral blood PD-1(+) CD8(+) T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin Cancer Res. 2019; 25:2144–54.27. Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017; 114:4993–8.28. Yamauchi T, Hoki T, Oba T, Jain V, Chen H, Attwood K, et al. T-cell CX3CR1 expression as a dynamic blood-based biomarker of response to immune checkpoint inhibitors. Nat Commun. 2021; 12:1402.
Article29. Chow A, Uddin FZ, Liu M, Dobrin A, Nabet BY, Mangarin L, et al. The ectonucleotidase CD39 identifies tumor-reactive CD8(+) T cells predictive of immune checkpoint blockade efficacy in human lung cancer. Immunity. 2023; 56:93–106.
Article30. Tsuboi M, Herbst RS, John T, Kato T, Majem M, Grohe C, et al. Overall survival with osimertinib in resected EGFR-mutated NSCLC. N Engl J Med. 2023; 389:137–47.
Article31. Park S, Kim TM, Han JY, Lee GW, Shim BY, Lee YG, et al. Phase III, randomized study of atezolizumab plus bevacizumab and chemotherapy in patients with EGFR- or ALK-mutated non-small-cell lung cancer (ATTLAS, KCSG-LU19-04). J Clin Oncol. 2024; 42:1241–51.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognostic Significance of Multiple Station N2 in Patients with Surgically Resected Stage IIIA N2 Non-small Cell Lung Cancer
- Risk Factor Analysis of Morbidity and 90-Day Mortality of Curative Resection in Patients with Stage IIIA–N2 Non-Small Cell Lung Cancer after Induction Concurrent Chemoradiation Therapy
- Adjuvant Chemotherapy for Completely Resected Non-Small Cell Lung Cancer
- Nodal Station as a Prognostic Factor in Resected Stage IIIA N2 Non-Small Cell Lung Cancer
- Early Result of Surgical Resection after Pre-Operative Concurrent chemoradiotherapy for N2-Positive Stage IIIA NSCLC