Nutr Res Pract.
2024 Oct;18(5):617-632. 10.4162/nrp.2024.18.5.617.
Dietary ellagic acid blocks inflammation-associated atherosclerotic plaque formation in cholesterol-fed apoE-deficient mice
- Affiliations
-
- 1Department of Food Science and Nutrition and Korean Institute of Nutrition, Hallym University, Chuncheon 24252, Korea
- 2Department of Food and Nutrition, Andong National University, Andong 36729, Korea
- KMID: 2560048
- DOI: http://doi.org/10.4162/nrp.2024.18.5.617
Abstract
- BACKGROUND/OBJECTIVES
Atherosclerosis particularly due to high circulating level of low-density lipoprotein is a major cause of cardiovascular diseases. Ellagic acid is a natural polyphenolic compound rich in pomegranates and berries. Our previous study showed that ellagic acid improved functionality of reverse cholesterol transport in murine model of atherosclerosis. The aim of this study is to investigate whether ellagic acid inhibited inflammation-associated atherosclerotic plaque formation in cholesterol-fed apolipoprotein E (apoE)-knockout (KO) mice.
MATERIALS/METHODS
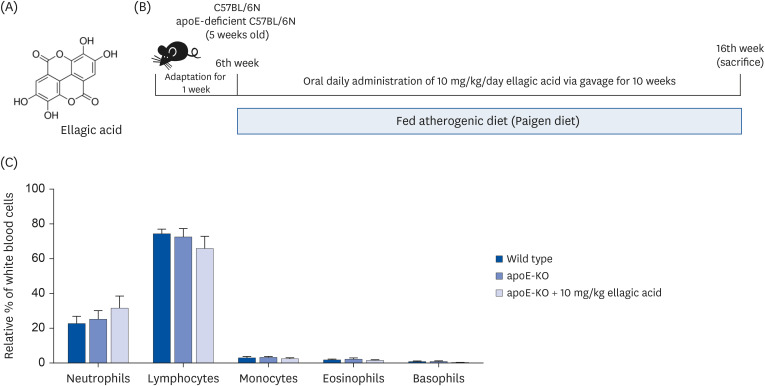
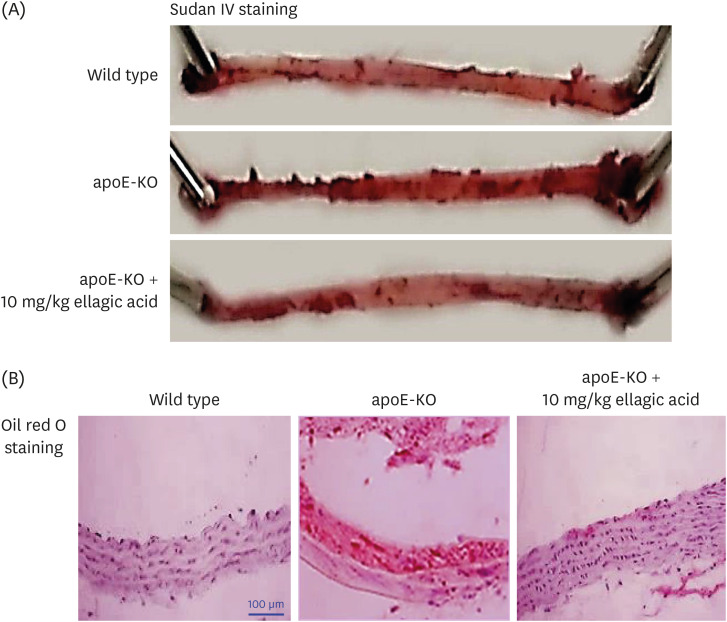
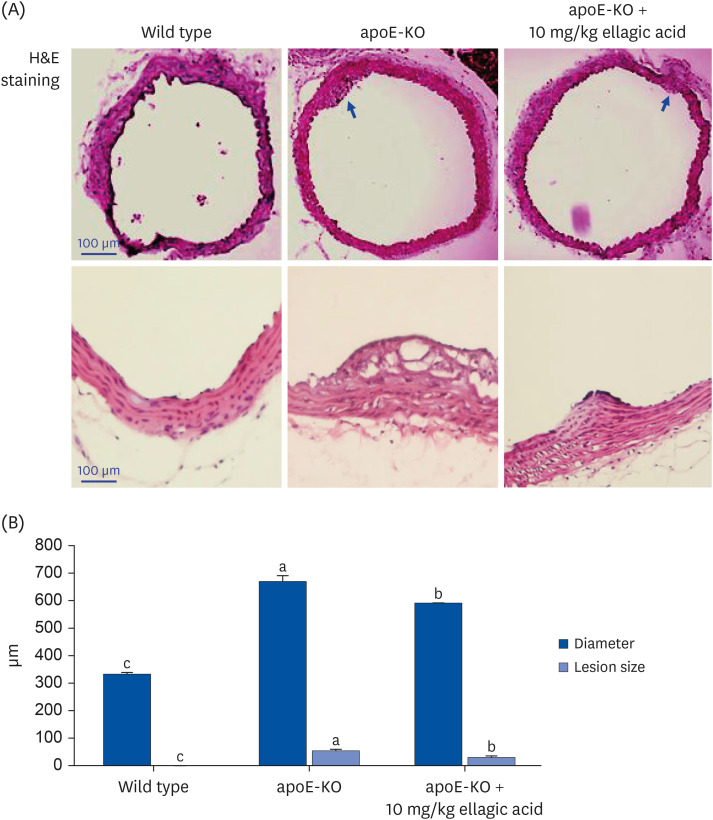
Wild type mice and apoE-KO mice were fed a cholesterol-rich Paigen diet for 10 weeks to induce severe atherosclerosis. Concurrently, 10 mg/kg ellagic acid was orally administered to the apoE-KO mice. Plaque lesion formation and lipid deposition were examined by staining with hematoxylin and eosin, Sudan IV and oil red O.
RESULTS
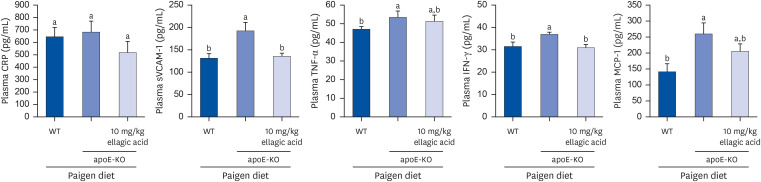
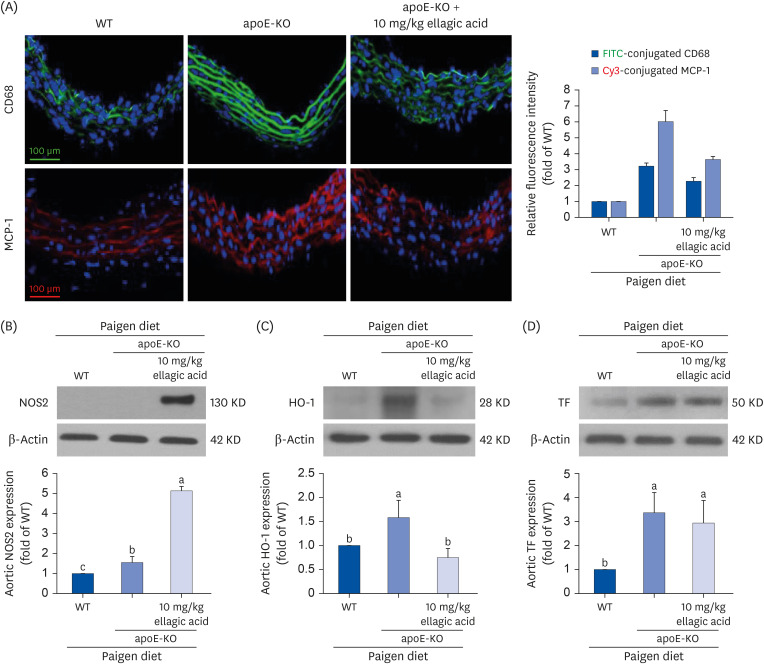
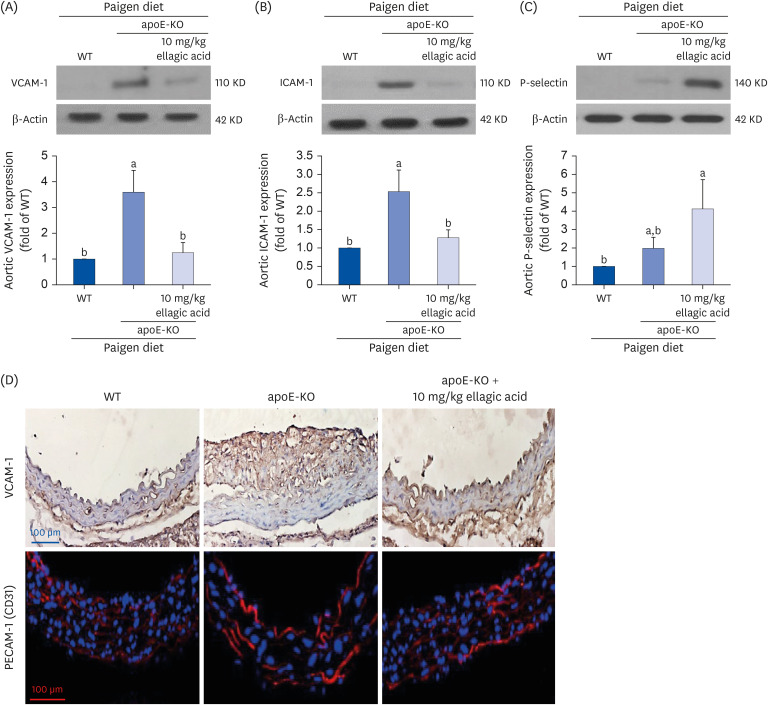
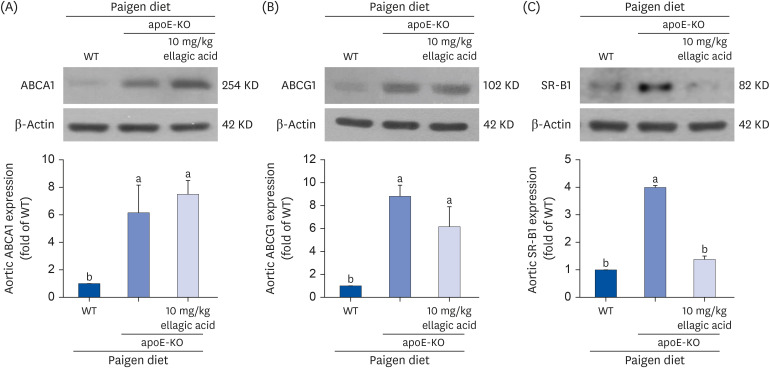
The plasma leukocyte profile of cholesterol-fed mice was not altered by apoE deficiency. Oral administration of ellagic acid attenuated plaque lesion formation and lipid deposition in the aorta tree of apoE-KO mice. Ellagic acid substantially reduced plasma levels of soluble vascular cell adhesion molecule and interferon-γ in Paigen diet-fed apoE-KO mice. When 10 mg/kg ellagic acid was administered to cholesterol-fed apoE-KO mice, the levels of CD68 and MCP-1 were strongly reduced in aorta vessels. The protein expression level of nitric oxide synthase-2 (NOS2) in the aorta was highly enhanced by supplementation of ellagic acid to apoE-KO mice, but the expression level of heme oxygenase-1 (HO-1) in the aorta was reduced. Furthermore, ellagic acid diminished the increased aorta expression of the inflammatory adhesion molecules in cholesterol-fed apoE-KO mice. The treatment of ellagic acid inhibited the scavenger receptor-B1 expression in the aorta of apoE-KO mice, while the cholesterol efflux-related transporters were not significantly changed.
CONCLUSION
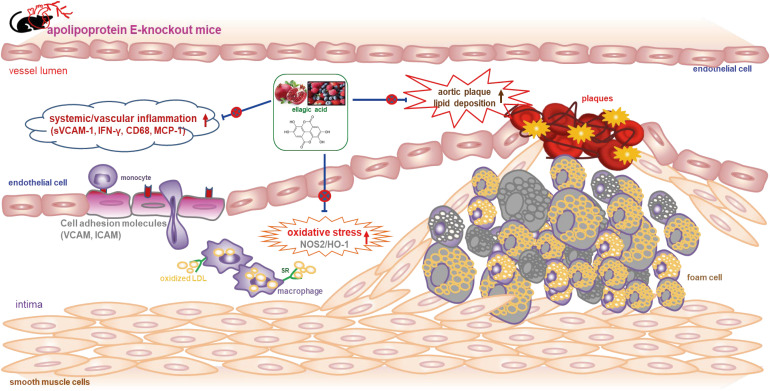
These results suggest that ellagic acid may be an atheroprotective compound by attenuating apoE deficiency-induced vascular inflammation and reducing atherosclerotic plaque lesion formation.
Figure
Reference
-
1. Fazio S, Linton MF. Mouse models of hyperlipidemia and atherosclerosis. Front Biosci. 2001; 6:D515–D525. PMID: 11229870.2. Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol. 2004; 24:1006–1014. PMID: 15087308.3. Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994; 14:133–140. PMID: 8274468.4. Lee YT, Lin HY, Chan YW, Li KH, To OT, Yan BP, Liu T, Li G, Wong WT, Keung W, et al. Mouse models of atherosclerosis: a historical perspective and recent advances. Lipids Health Dis. 2017; 16:12. PMID: 28095860.5. Mahdinia E, Shokri N, Taheri AT, Asgharzadeh S, Elahimanesh M, Najafi M. Cellular crosstalk in atherosclerotic plaque microenvironment. Cell Commun Signal. 2023; 21:125. PMID: 37254185.6. Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014; 5:927–946. PMID: 25489440.7. Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, Benito-Vicente A, Martín C. Pathophysiology of atherosclerosis. Int J Mol Sci. 2022; 23:3346. PMID: 35328769.8. Mehu M, Narasimhulu CA, Singla DK. Inflammatory cells in atherosclerosis. Antioxidants. 2022; 11:233. PMID: 35204116.9. Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016; 118:692–702. PMID: 26892967.10. Johnson JL, Jackson CL. Atherosclerotic plaque rupture in the apolipoprotein E knockout mouse. Atherosclerosis. 2001; 154:399–406. PMID: 11166772.11. Jawien J. The role of an experimental model of atherosclerosis: apoE-knockout mice in developing new drugs against atherogenesis. Curr Pharm Biotechnol. 2012; 13:2435–2439. PMID: 22280417.12. Kobiyama K, Ley K. Atherosclerosis. Circ Res. 2018; 123:1118–1120. PMID: 30359201.13. Katsuda S, Kaji T. Atherosclerosis and extracellular matrix. J Atheroscler Thromb. 2003; 10:267–274. PMID: 14718743.14. Farahi L, Sinha SK, Lusis AJ. Roles of macrophages in atherogenesis. Front Pharmacol. 2021; 12:785220. PMID: 34899348.15. Lee-Rueckert M, Lappalainen J, Kovanen PT, Escola-Gil JC. Lipid-laden macrophages and inflammation in atherosclerosis and cancer: an integrative view. Front Cardiovasc Med. 2022; 9:777822. PMID: 35237673.16. Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ, Han M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. 2022; 7:131. PMID: 35459215.17. Engelen SE, Robinson AJ, Zurke YX, Monaco C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat Rev Cardiol. 2022; 19:522–542. PMID: 35102320.18. Li B, Li W, Li X, Zhou H. Inflammation: a novel therapeutic target/direction in atherosclerosis. Curr Pharm Des. 2017; 23:1216–1227. PMID: 28034355.19. Ziółkiewicz A, Kasprzak-Drozd K, Rusinek R, Markut-Miotła E, Oniszczuk A. The Influence of polyphenols on atherosclerosis development. Int J Mol Sci. 2023; 24:7146. PMID: 37108307.20. de Araújo FF, de Paulo Farias D, Neri-Numa IA, Pastore GM. Polyphenols and their applications: an approach in food chemistry and innovation potential. Food Chem. 2021; 338:127535. PMID: 32798817.21. Lee WJ, Ou HC, Hsu WC, Chou MM, Tseng JJ, Hsu SL, Tsai KL, Sheu WH. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J Vasc Surg. 2010; 52:1290–1300. PMID: 20692795.22. Larrosa M, García-Conesa MT, Espín JC, Tomás-Barberán FA. Ellagitannins, ellagic acid and vascular health. Mol Aspects Med. 2010; 31:513–539. PMID: 20837052.23. Park SH, Kim JL, Lee ES, Han SY, Gong JH, Kang MK, Kang YH. Dietary ellagic acid attenuates oxidized LDL uptake and stimulates cholesterol efflux in murine macrophages. J Nutr. 2011; 141:1931–1937. PMID: 21940512.24. Lawrie A, Hameed AG, Chamberlain J, Arnold N, Kennerley A, Hopkinson K, Pickworth J, Kiely DG, Crossman DC, Francis SE. Paigen diet-fed apolipoprotein E knockout mice develop severe pulmonary hypertension in an interleukin-1-dependent manner. Am J Pathol. 2011; 179:1693–1705. PMID: 21835155.25. Getz GS, Reardon CA. Diet and murine atherosclerosis. Arterioscler Thromb Vasc Biol. 2006; 26:242–249. PMID: 16373607.26. Miyoshi T, Li Y, Shih DM, Wang X, Laubach VE, Matsumoto AH, Helm GA, Lusis AJ, Shi W. Deficiency of inducible NO synthase reduces advanced but not early atherosclerosis in apolipoprotein E-deficient mice. Life Sci. 2006; 79:525–531. PMID: 16516241.27. Detmers PA, Hernandez M, Mudgett J, Hassing H, Burton C, Mundt S, Chun S, Fletcher D, Card DJ, Lisnock J, et al. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J Immunol. 2000; 165:3430–3435. PMID: 10975863.28. Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the apoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998; 18:842–851. PMID: 9598845.29. Mu W, Chen M, Gong Z, Zheng F, Xing Q. Expression of vascular cell adhesion molecule-1 in the aortic tissues of atherosclerotic patients and the associated clinical implications. Exp Ther Med. 2015; 10:423–428. PMID: 26622332.30. Joyce CW, Amar MJ, Lambert G, Vaisman BL, Paigen B, Najib-Fruchart J, Hoyt RF Jr, Neufeld ED, Remaley AT, Fredrickson DS, et al. The ATP binding cassette transporter A1 (ABCA1) modulates the development of aortic atherosclerosis in C57BL/6 and apoE-knockout mice. Proc Natl Acad Sci U S A. 2002; 99:407–412. PMID: 11752403.31. Zadelaar S, Kleemann R, Verschuren L, de Vries-Van der Weij J, van der Hoorn J, Princen HM, Kooistra T. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol. 2007; 27:1706–1721. PMID: 17541027.32. Curtiss LK. ApoE in atherosclerosis : a protein with multiple hats. Arterioscler Thromb Vasc Biol. 2000; 20:1852–1853. PMID: 10938002.33. Ilhan F, Kalkanli ST. Atherosclerosis and the role of immune cells. World J Clin Cases. 2015; 3:345–352. PMID: 25879006.34. Voloshyna I, Littlefield MJ, Reiss AB. Atherosclerosis and interferon-γ: new insights and therapeutic targets. Trends Cardiovasc Med. 2014; 24:45–51. PMID: 23916809.35. Yamashita T, Sasaki N, Kasahara K, Hirata K. Anti-inflammatory and immune-modulatory therapies for preventing atherosclerotic cardiovascular disease. J Cardiol. 2015; 66:1–8. PMID: 25744783.36. Hedin U, Matic LP. Recent advances in therapeutic targeting of inflammation in atherosclerosis. J Vasc Surg. 2019; 69:944–951. PMID: 30591299.37. Loke WM, Proudfoot JM, Hodgson JM, McKinley AJ, Hime N, Magat M, Stocker R, Croft KD. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2010; 30:749–757. PMID: 20093625.38. Park SH, Sim YE, Kang MK, Kim DY, Kang IJ, Lim SS, Kang YH. Purple perilla frutescens extracts containing α-asarone inhibit inflammatory atheroma formation and promote hepatic HDL cholesterol uptake in dyslipidemic apoE-deficient mice. Nutr Res Pract. 2023; 17:1099–1112. PMID: 38053825.39. Park SH, Kang MK, Kim DY, Lim SS, Kang IJ, Kang YH. Ellagic acid, a functional food component, ameliorates functionality of reverse cholesterol transport in murine model of atherosclerosis. Nutr Res Pract. 2024; 18:194–209. PMID: 38584811.40. Campbell NK, Fitzgerald HK, Dunne A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat Rev Immunol. 2021; 21:411–425. PMID: 33514947.41. Ulyanova T, Szél A, Kutty RK, Wiggert B, Caffé AR, Chader GJ, van Veen T. Oxidative stress induces heme oxygenase-1 immunoreactivity in Müller cells of mouse retina in organ culture. Invest Ophthalmol Vis Sci. 2001; 42:1370–1374. PMID: 11328753.42. Chiang SK, Chen SE, Chang LC. The role of HO-1 and its crosstalk with oxidative stress in cancer cell survival. Cells. 2021; 10:2401. PMID: 34572050.43. Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013; 13:709–721. PMID: 23995626.44. Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007; 116:1832–1844. PMID: 17938300.45. Huang L, Chambliss KL, Gao X, Yuhanna IS, Behling-Kelly E, Bergaya S, Ahmed M, Michaely P, Luby-Phelps K, Darehshouri A, et al. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature. 2019; 569:565–569. PMID: 31019307.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ellagic acid, a functional food component, ameliorates functionality of reverse cholesterol transport in murine model of atherosclerosis
- Purple perilla frutescens extracts containing α-asarone inhibit inflammatory atheroma formation and promote hepatic HDL cholesterol uptake in dyslipidemic apoE-deficient mice
- Toxicity and efficacy study of a combination of two retinoic acids in an ApoE knockout mouse model of atherosclerosis
- Spatial and Temporal Expression, and Statin Responsiveness of Galectin-1 and Galectin-3 in Murine Atherosclerosis
- Effect of Human Bcl-2 Gene Expression on the Peripheral Atherosclerotic Lesions of Apolipoprotein E-Deficient Mouse