J Rheum Dis.
2024 Oct;31(4):212-222. 10.4078/jrd.2024.0026.
Anemia as an indicator of a higher retention rate for tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis from a Korean multicenter registry
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
- KMID: 2559890
- DOI: http://doi.org/10.4078/jrd.2024.0026
Abstract
Objective
To examine whether simple laboratory tests can guide selection between tocilizumab (TCZ) and tumor necrosis factor inhibitors (TNFi) in biologic-naive patients with rheumatoid arthritis (RA), by investigating their influence on drug retention.
Methods
Data of RA patients prescribed TCZ or TNFi as the initial biologics from March 2013 to December 2021 were obtained from the KOrean College of Rheumatology BIOlogics and Targeted Therapy (KOBIO) registry. Propensity score matching was performed to adjust for baseline confounding factors. Hazards of drug discontinuation for TCZ were calculated compared to those for TNFi. Interaction analyses with a Bonferroni-corrected p-value threshold were conducted to determine whether the hemoglobin level, C-reactive protein level, erythrocyte sedimentation rate, and platelet count affected the hazards of drug discontinuation.
Results
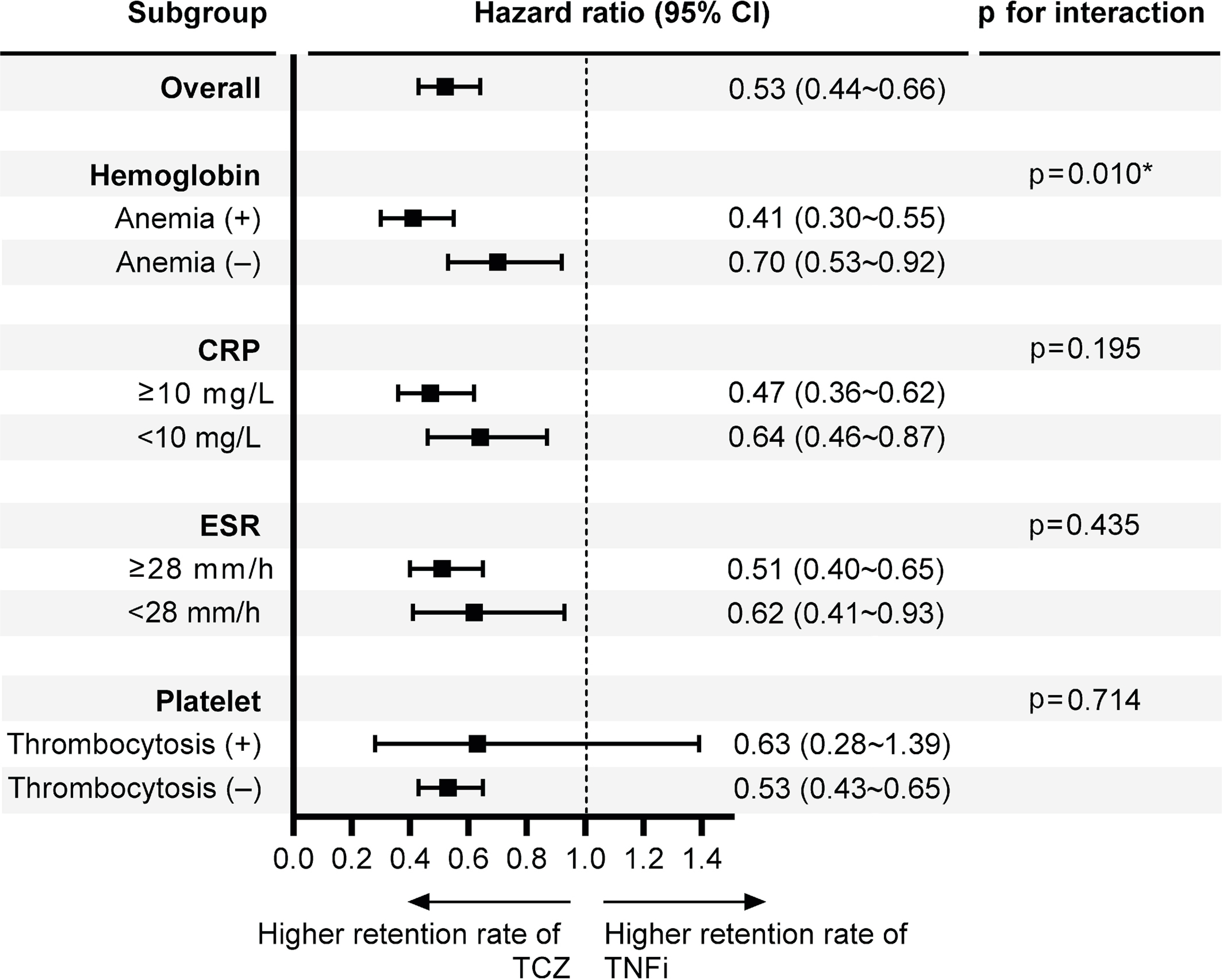
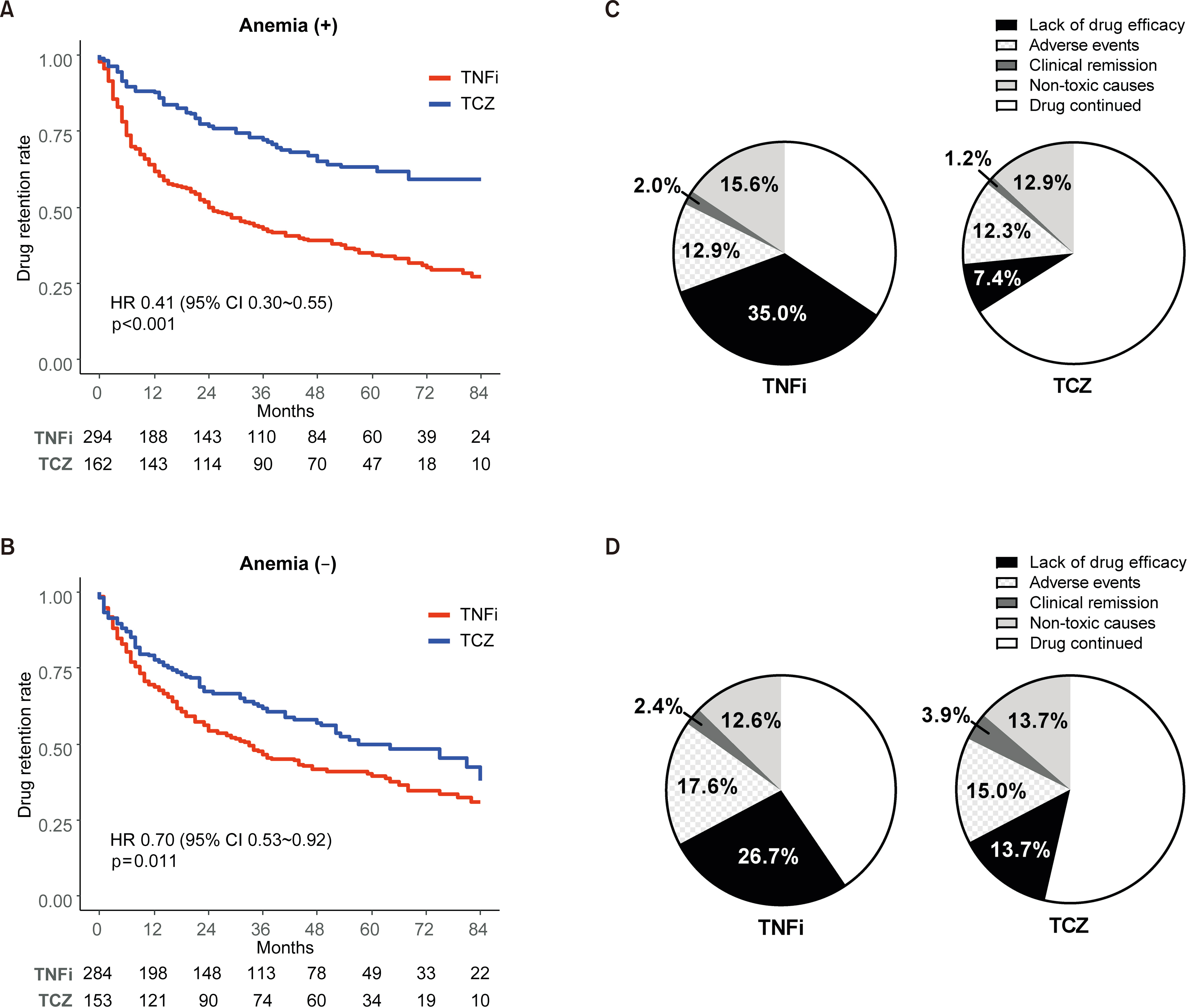
Overall, 893 patients were analyzed, of whom 315 and 578 were treated with TCZ and TNFi, respectively. The hazards of drug discontinuation in all patients were lower for TCZ than for TNFi (hazard ratio [HR]: 0.53, 95% confidence interval [CI]: 0.44~0.66). Notably, only the presence of anemia indicated a significant interaction (p for interaction=0.010); the HRs for drug discontinuation were 0.41 (95% CI: 0.30~0.55) and 0.70 (95% CI: 0.53~0.92) in the anemic and non-anemic groups, respectively. In the anemic subgroup, biologics were discontinued because of a lack of efficacy in 35.0% of TNFi initiators and 7.4% of TCZ initiators.
Conclusion
The drug discontinuation rate in biologic-naïve patients with RA was significantly lower for TCZ than for TNFi, particularly in those with anemia.
Figure
Reference
-
1. Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. 2018; Rheumatoid arthritis. Nat Rev Dis Primers. 4:18001. DOI: 10.1038/nrdp.2018.1. PMID: 29417936.2. Smolen JS, Landewé RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, et al. 2023; EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 82:3–18. DOI: 10.1136/ard-2022-223356. PMID: 36357155.3. Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, et al. 2021; 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 73:924–39. DOI: 10.1002/acr.24596. PMID: 34101387. PMCID: PMC9273041.4. Emery P, Gottenberg JE, Rubbert-Roth A, Sarzi-Puttini P, Choquette D, Taboada VM, et al. 2015; Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 74:979–84. DOI: 10.1136/annrheumdis-2013-203993. PMID: 24442884. PMCID: PMC4431330.5. Schett G, McInnes IB, Neurath MF. 2021; Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med. 385:628–39. DOI: 10.1056/NEJMra1909094. PMID: 34379924.6. Tanaka T, Narazaki M, Kishimoto T. 2014; IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 6:a016295. DOI: 10.1101/cshperspect.a016295. PMID: 25190079. PMCID: PMC4176007.7. Boyapati A, Schwartzman S, Msihid J, Choy E, Genovese MC, Burmester GR, et al. 2020; Association of high serum interleukin-6 levels with severe progression of rheumatoid arthritis and increased treatment response differentiating sarilumab from adalimumab or methotrexate in a post hoc analysis. Arthritis Rheumatol. 72:1456–66. DOI: 10.1002/art.41299. PMID: 32343882. PMCID: PMC7496495.8. Kaneko S, Shimizu M, Miyaoka F, Shimbo A, Irabu H, Mizuta M, et al. 2023; The dynamics of laboratory markers reflecting cytokine overproduction in macrophage activation syndrome complicated with systemic juvenile idiopathic arthritis. Clin Immunol. 248:109270. DOI: 10.1016/j.clim.2023.109270. PMID: 36806704.9. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. 2004; IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 113:1271–6. DOI: 10.1172/JCI200420945. PMID: 36996433.10. Raj DS. 2009; Role of interleukin-6 in the anemia of chronic disease. Semin Arthritis Rheum. 38:382–8. DOI: 10.1016/j.semarthrit.2008.01.006. PMID: 18336871.11. Ganapathi MK, May LT, Schultz D, Brabenec A, Weinstein J, Sehgal PB, et al. 1988; Role of interleukin-6 in regulating synthesis of C-reactive protein and serum amyloid A in human hepatoma cell lines. Biochem Biophys Res Commun. 157:271–7. DOI: 10.1016/S0006-291X(88)80043-3. PMID: 2461709.12. Castell JV, Gómez-Lechón MJ, David M, Andus T, Geiger T, Trullenque R, et al. 1989; Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 242:237–9. DOI: 10.1016/0014-5793(89)80476-4. PMID: 2464504.13. Ishibashi T, Kimura H, Shikama Y, Uchida T, Kariyone S, Hirano T, et al. 1989; Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood. 74:1241–4. DOI: 10.1182/blood.V74.4.1241.1241. PMID: 2788464.14. Ishibashi T, Kimura H, Uchida T, Kariyone S, Friese P, Burstein SA. 1989; Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci U S A. 86:5953–7. DOI: 10.1073/pnas.86.15.5953. PMID: 2788282. PMCID: PMC297749.15. Boss B, Neeck G. 2000; Correlation of IL-6 with the classical humoral disease activity parameters ESR and CRP and with serum cortisol, reflecting the activity of the HPA axis in active rheumatoid arthritis. Z Rheumatol. 59(Suppl 2):II/62-4. DOI: 10.1007/s003930070020. PMID: 11155806.16. Milovanovic M, Nilsson E, Järemo P. 2004; Relationships between platelets and inflammatory markers in rheumatoid arthritis. Clin Chim Acta. 343:237–40. DOI: 10.1016/j.cccn.2003.12.030. PMID: 15115702.17. Nikolaisen C, Figenschau Y, Nossent JC. 2008; Anemia in early rheumatoid arthritis is associated with interleukin 6-mediated bone marrow suppression, but has no effect on disease course or mortality. J Rheumatol. 35:380–6. PMID: 18260177.18. van Leeuwen MA, Westra J, Limburg PC, van Riel PL, van Rijswijk MH. 1995; Clinical significance of interleukin-6 measurement in early rheumatoid arthritis: relation with laboratory and clinical variables and radiological progression in a three year prospective study. Ann Rheum Dis. 54:674–7. DOI: 10.1136/ard.54.8.674. PMID: 7677445. PMCID: PMC1009966.19. Nakagawa J, Koyama Y, Kawakami A, Ueki Y, Tsukamoto H, Horiuchi T, et al. 2017; A novel scoring system based on common laboratory tests predicts the efficacy of TNF-inhibitor and IL-6 targeted therapy in patients with rheumatoid arthritis: a retrospective, multicenter observational study. Arthritis Res Ther. 19:185. DOI: 10.1186/s13075-017-1387-9. PMID: 28800780. PMCID: PMC5553584.20. Kim J, Koh JH, Choi SJ, Jeon CH, Kwok SK, Kim SK, et al. 2021; KOBIO, the first web-based Korean biologics registry operated with a unified platform among distinct disease entities. J Rheum Dis. 28:176–82. DOI: 10.4078/jrd.2021.28.4.176. PMID: 37476366. PMCID: PMC10324910.21. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010; 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62:2569–81. DOI: 10.1002/art.27584. PMID: 20872595.22. World Health Organization. 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. World Health Organization;Geneva:23. Macy EM, Hayes TE, Tracy RP. 1997; Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 43:52–8. DOI: 10.1093/clinchem/43.1.52. PMID: 8990222.24. Sokka T, Pincus T. 2003; Most patients receiving routine care for rheumatoid arthritis in 2001 did not meet inclusion criteria for most recent clinical trials or american college of rheumatology criteria for remission. J Rheumatol. 30:1138–46. PMID: 12784382.25. Skoda RC. 2009; Thrombocytosis. Hematology Am Soc Hematol Educ Program. 2009:159–67. DOI: 10.1182/asheducation-2009.1.159. PMID: 33275673. PMCID: PMC7727594.26. Lauper K, Nordström DC, Pavelka K, Hernández MV, Kvien TK, Kristianslund EK, et al. 2018; Comparative effectiveness of tocilizumab versus TNF inhibitors as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis after the use of at least one biologic disease-modifying antirheumatic drug: analyses from the pan-European TOCERRA register collaboration. Ann Rheum Dis. 77:1276–82. DOI: 10.1136/annrheumdis-2017-212845. PMID: 29730637.27. Lauper K, Mongin D, Iannone F, Kristianslund EK, Kvien TK, Nordström DC, et al. 2020; Comparative effectiveness of TNF inhibitors and tocilizumab with and without conventional synthetic disease-modifying antirheumatic drugs in a pan-European observational cohort of bio-naïve patients with rheumatoid arthritis. Semin Arthritis Rheum. 50:17–24. DOI: 10.1016/j.semarthrit.2019.06.020. PMID: 31280937.28. Nakayama Y, Watanabe R, Yamamoto W, Ebina K, Hirano T, Kotani T, et al. 2024; IL-6 inhibitors and JAK inhibitors as favourable treatment options for patients with anaemia and rheumatoid arthritis: ANSWER cohort study. Rheumatology (Oxford). 63:349–57. DOI: 10.1093/rheumatology/kead299. PMID: 37354495.29. Ebina K, Hashimoto M, Yamamoto W, Ohnishi A, Kabata D, Hirano T, et al. 2018; Drug retention and discontinuation reasons between seven biologics in patients with rheumatoid arthritis -the ANSWER cohort study. PLoS One. 13:e0194130. DOI: 10.1371/journal.pone.0194130. PMID: 29543846. PMCID: PMC5854351.30. Jinno S, Onishi A, Dubreuil M, Hashimoto M, Yamamoto W, Murata K, et al. 2021; Comparison of the drug retention and reasons for discontinuation of tumor necrosis factor inhibitors and interleukin-6 inhibitors in Japanese patients with elderly-onset rheumatoid arthritis-the ANSWER cohort study. Arthritis Res Ther. 23:116. DOI: 10.1186/s13075-021-02496-w. PMID: 33858490. PMCID: PMC8048332.31. Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, et al. 2002; Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 99:4596–601. DOI: 10.1073/pnas.072632499. PMID: 11930010. PMCID: PMC123693.32. Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, et al. 2001; Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 98:8780–5. DOI: 10.1073/pnas.151179498. PMID: 11447267. PMCID: PMC37512.33. Song SN, Iwahashi M, Tomosugi N, Uno K, Yamana J, Yamana S, et al. 2013; Comparative evaluation of the effects of treatment with tocilizumab and TNF-α inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther. 15:R141. DOI: 10.1186/ar4323. PMID: 24286116. PMCID: PMC3978580.34. Voulgari PV, Kolios G, Papadopoulos GK, Katsaraki A, Seferiadis K, Drosos AA. 1999; Role of cytokines in the pathogenesis of anemia of chronic disease in rheumatoid arthritis. Clin Immunol. 92:153–60. DOI: 10.1006/clim.1999.4736. PMID: 10444359.35. Papadaki HA, Kritikos HD, Valatas V, Boumpas DT, Eliopoulos GD. 2002; Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood. 100:474–82. DOI: 10.1182/blood-2002-01-0136. PMID: 12091338.36. Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. 2003; Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 101:2461–3. DOI: 10.1182/blood-2002-10-3235. PMID: 12433676.37. Nakayama Y, Hashimoto M, Watanabe R, Murakami K, Murata K, Tanaka M, et al. 2022; Favorable clinical response and drug retention of anti-IL-6 receptor inhibitor in rheumatoid arthritis with high CRP levels: the ANSWER cohort study. Scand J Rheumatol. 51:431–40. DOI: 10.1080/03009742.2021.1947005. PMID: 34511031.38. Wang J, Devenport J, Low JM, Yu D, Hitraya E. 2016; Relationship between baseline and early changes in C-reactive protein and interleukin-6 levels and clinical response to tocilizumab in rheumatoid arthritis. Arthritis Care Res (Hoboken). 68:882–5. DOI: 10.1002/acr.22765. PMID: 26473986. PMCID: PMC6120137.39. Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. 2000; Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 31:149–59. DOI: 10.1002/hep.510310123. PMID: 10613740.40. Ferreira JC, Patino CM. 2017; Subgroup analysis and interaction tests: why they are important and how to avoid common mistakes. J Bras Pneumol. 43:162. DOI: 10.1590/s1806-37562017000000170. PMID: 28746525. PMCID: PMC5687944.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Drug retention of biologic and targeted synthetic disease-modifying antirheumatic drugs in Korean patients with seropositive rheumatoid arthritis
- Description of the Efficacy and Safety of Three New Biologics in the Treatment of Rheumatoid Arthritis
- Effect of Tocilizumab on Serum Hepcidin and Anemia Response in Patients with Rheumatoid Arthritis
- Review of Tumor Necrosis Factor Inhibitors on Rheumatoid Arthritis
- Interstitial pneumonitis associated with infliximab therapy in a rheumatoid arthritis patient