Anat Cell Biol.
2024 Sep;57(3):408-418. 10.5115/acb.24.120.
Aptamin C enhances anti-cancer activity NK cells through the activation of STAT3: a comparative study with vitamin C
- Affiliations
-
- 1Laboratory of Vitamin C and Antioxidant Immunology, Department of Anatomy and Cell Biology, Seoul National University College of Medicine, Seoul, Korea
- 2Institute of Allergy and Clinical Immunology, Medical Research Center, Seoul National University, Seoul, Korea
- 3Department of Research and Development, N Therapeutics Co., Ltd., Seoul, 4 Nexmos, Inc., Yongin, Korea
- 4Department of Applied Bioengineering, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Korea
- 5Artificial Intelligence Institute, Seoul National University, Seoul, Korea
- KMID: 2559759
- DOI: http://doi.org/10.5115/acb.24.120
Abstract
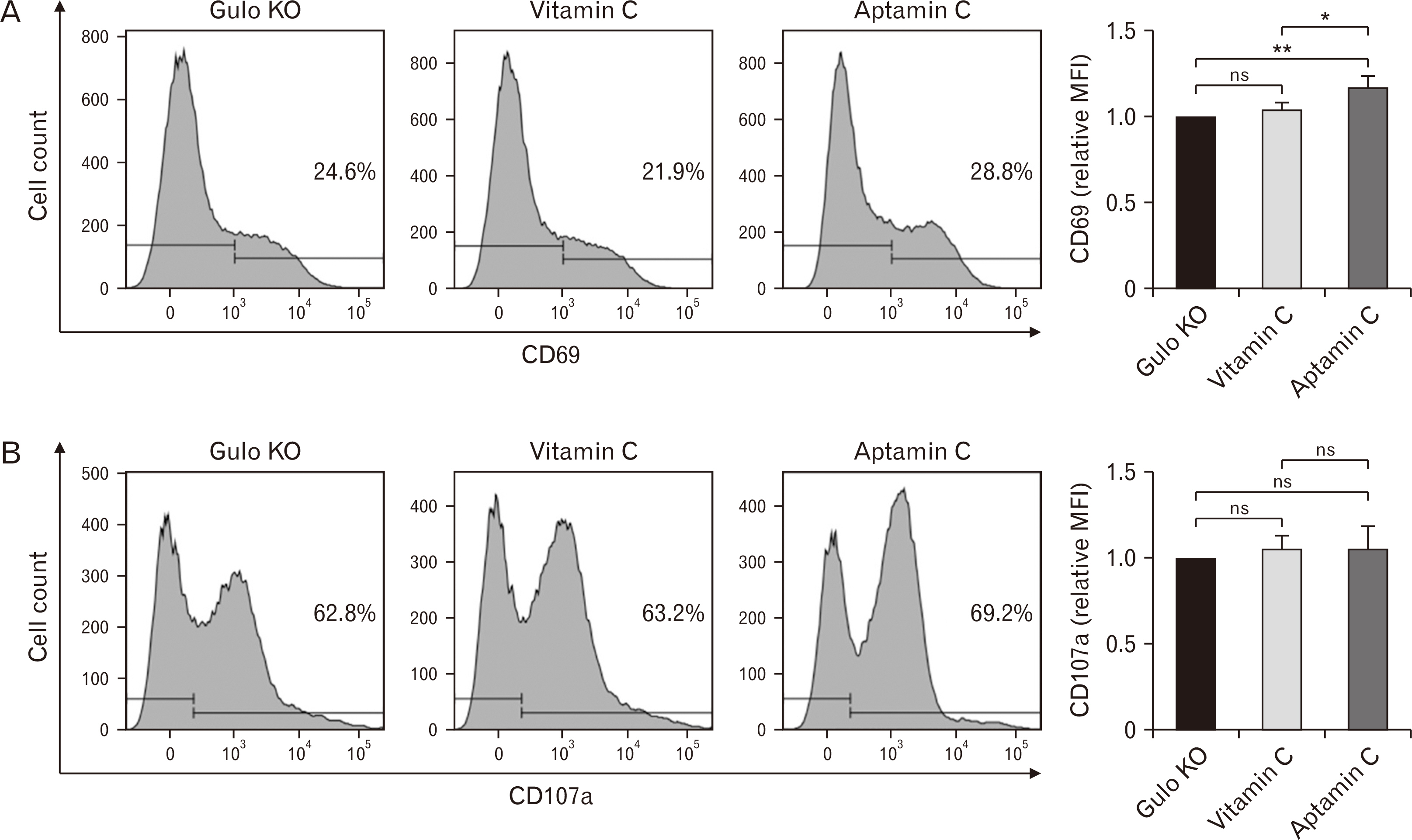
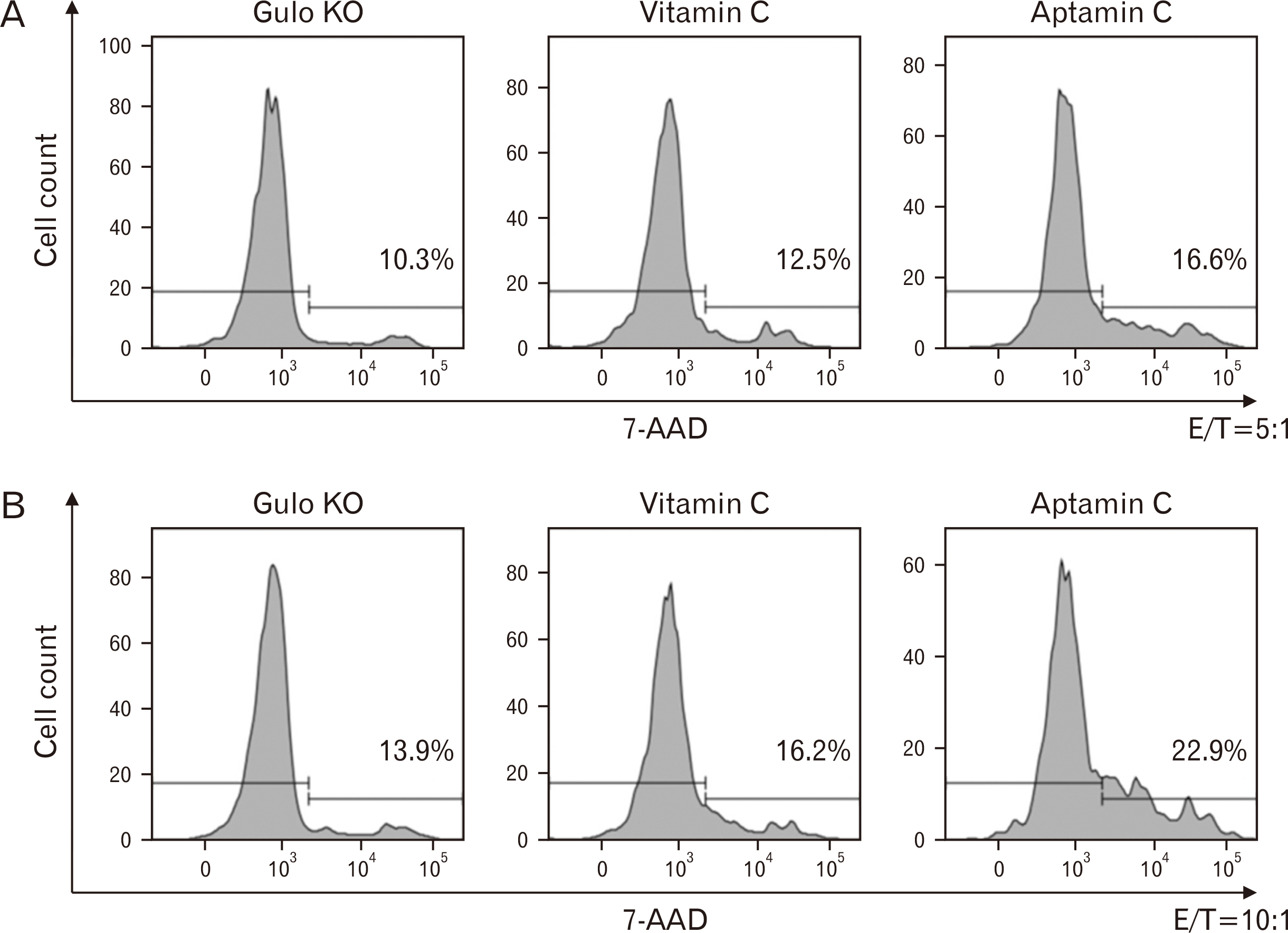
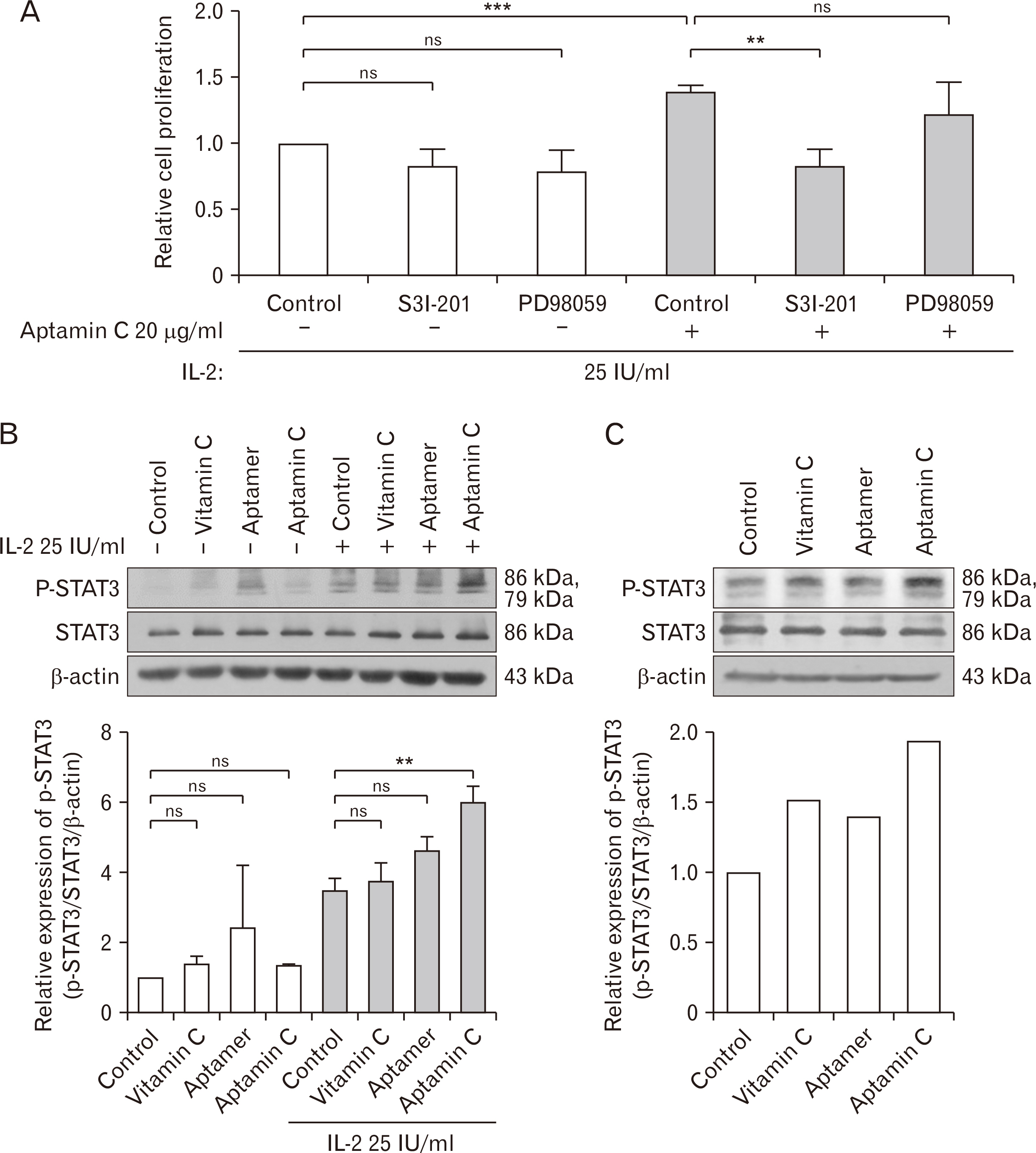
- Vitamin C is a well-known antioxidant with antiviral, anticancer, and anti-inflammatory properties based on its antioxidative function. Aptamin C, a complex of vitamin C with its specific aptamer, has been reported to maintain or even enhance the efficacy of vitamin C while increasing its stability. To investigate in vivo distribution of Aptamin C, Gulo knockout mice, which, like humans, cannot biosynthesize vitamin C, were administered Aptamin C orally for 2 and 4 weeks. The results showed higher vitamin C accumulation in all tissues when administered Aptamin C, especially in the spleen. Next, the activity of natural killer (NK) cells were conducted. CD69, a marker known for activating for NK cells, which had decreased due to vitamin C deficiency, did not recover with vitamin C treatment but showed an increasing with Aptamin C. Furthermore, the expression of CD107a, a cell surface marker that increases during the killing process of target cells, also did not recover with vitamin C but increased with Aptamin C. Based on these results, when cultured with tumor cells to measure the extent of tumor cell death, an increase in tumor cell death was observed. To investigate the signaling mechanisms and related molecules involved in the proliferation and activation of NK cells by Aptamin C showed that Aptamin C treatment led to an increase in intracellular STAT3 activation. In conclusion, Aptamin C has a higher capability to activate NK cells and induce tumor cell death compared to vitamin C and it is mediated through the activation of STAT3.
Figure
Reference
-
References
1. Caritá AC, Fonseca-Santos B, Shultz JD, Michniak-Kohn B, Chorilli M, Leonardi GR. 2020; Vitamin C: one compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine. 24:102117. DOI: 10.1016/j.nano.2019.102117. PMID: 31676375.2. Meščić Macan A, Gazivoda Kraljević T, Raić-Malić S. 2019; Therapeutic perspective of vitamin C and its derivatives. Antioxidants (Basel). 8:247. DOI: 10.3390/antiox8080247. PMID: 31357509. PMCID: PMC6721080.3. Takamizawa S, Maehata Y, Imai K, Senoo H, Sato S, Hata R. 2004; Effects of ascorbic acid and ascorbic acid 2-phosphate, a long-acting vitamin C derivative, on the proliferation and differentiation of human osteoblast-like cells. Cell Biol Int. 28:255–65. DOI: 10.1016/j.cellbi.2004.01.010. PMID: 15109981.4. Campos PM, Gonçalves GM, Gaspar LR. 2008; In vitro antioxidant activity and in vivo efficacy of topical formulations containing vitamin C and its derivatives studied by non-invasive methods. Skin Res Technol. 14:376–80. DOI: 10.1111/j.1600-0846.2008.00288.x. PMID: 19159387.5. Swindell WR, Randhawa M, Quijas G, Bojanowski K, Chaudhuri RK. 2021; Tetrahexyldecyl ascorbate (THDC) degrades rapidly under oxidative stress but can be stabilized by acetyl zingerone to enhance collagen production and antioxidant effects. Int J Mol Sci. 22:8756. DOI: 10.3390/ijms22168756. PMID: 34445461. PMCID: PMC8395926.6. Yamamoto I, Tai A, Fujinami Y, Sasaki K, Okazaki S. 2002; Synthesis and characterization of a series of novel monoacylated ascorbic acid derivatives, 6-O-acyl-2-O-alpha-D-glucopyranosyl-L-ascorbic acids, as skin antioxidants. J Med Chem. 45:462–8. DOI: 10.1021/jm010379f. PMID: 11784150.7. Ravetti S, Clemente C, Brignone S, Hergert L, Allemandi D, Palma S. 2019; Ascorbic acid in skin health. Cosmetics. 6:58. DOI: 10.3390/cosmetics6040058.8. Weeks BS, Perez PP. 2007; Absorption rates and free radical scavenging values of vitamin C-lipid metabolites in human lymphoblastic cells. Med Sci Monit. 13:BR205–10. PMID: 17901843.9. Pinnell SR, Yang H, Omar M, Monteiro-Riviere N, DeBuys HV, Walker LC, Wang Y, Levine M. 2001; Topical L-ascorbic acid: percutaneous absorption studies. Dermatol Surg. 27:137–42. DOI: 10.1097/00042728-200102000-00008. PMID: 11207686.10. Song MK, Lee JH, Kim J, Kim JH, Hwang S, Kim YS, Kim YJ. 2021; Neuroprotective effect of NXP031 in the MPTP-induced Parkinson's disease model. Neurosci Lett. 740:135425. DOI: 10.1016/j.neulet.2020.135425. PMID: 33075422.11. Choi S, Han J, Kim JH, Kim AR, Kim SH, Lee W, Yoon MY, Kim G, Kim YS. 2020; Advances in dermatology using DNA aptamer "Aptamin C" innovation: oxidative stress prevention and effect maximization of vitamin C through antioxidation. J Cosmet Dermatol. 19:970–6. DOI: 10.1111/jocd.13081. PMID: 31353789. PMCID: PMC7154658.12. Lee D, Kim Y, Jo H, Go C, Jeong Y, Jang Y, Kang D, Park K, Kim YS, Kang JS. 2021; The anti-inflammatory effect of Aptamin C on house dust mite extract-induced inflammation in keratinocytes via regulation of IL-22 and GDNF production. Antioxidants (Basel). 10:945. DOI: 10.3390/antiox10060945. PMID: 34208021. PMCID: PMC8230602.13. Kong AH, Wu AJ, Ho OK, Leung MM, Huang AS, Yu Y, Zhang G, Lyu A, Li M, Cheung KH. 2023; Exploring the potential of aptamers in targeting neuroinflammation and neurodegenerative disorders: opportunities and challenges. Int J Mol Sci. 24:11780. DOI: 10.3390/ijms241411780. PMID: 37511539. PMCID: PMC10380291.14. Delves PJ. Rose NR, Mackay IR, editors. Innate and adaptive systems of immunity. The Autoimmune Diseases. 6th ed. Academic Press;2020. p. 45–61. DOI: 10.1016/B978-0-12-812102-3.00004-X.15. Vivier E, Malissen B. 2005; Innate and adaptive immunity: specificities and signaling hierarchies revisited. Nat Immunol. 6:17–21. DOI: 10.1038/ni1153. PMID: 15611777. PMCID: PMC7097365.16. Khan MM. Khan MM, editor. Overview of the immune response. Immunopharmacology. Springer;2016. p. 1–55. DOI: 10.1007/978-3-319-30273-7_1.17. Nutt SL, Huntington ND. Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM, editors. Cytotoxic T lymphocytes and natural killer cells. Clinical Immunology. 5th ed. Elsevier;2019. p. 247–59.e1. DOI: 10.1016/B978-0-7020-6896-6.00017-X.18. Guglielmo A, Zengarini C, Agostinelli C, Motta G, Sabattini E, Pileri A. 2024; The role of cytokines in cutaneous T cell lymphoma: a focus on the state of the art and possible therapeutic targets. Cells. 13:584. DOI: 10.3390/cells13070584. PMID: 38607023. PMCID: PMC11012008.19. Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O'Mahony L, Palomares O, Rhyner C, Ouaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, Akdis CA. 2011; Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 127:701–21. e1–70. DOI: 10.1016/j.jaci.2010.11.050. PMID: 21377040.20. Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Röcken M. 1994; Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 180:1961–6. DOI: 10.1084/jem.180.5.1961. PMID: 7525845. PMCID: PMC2191757.21. Yoshimoto T, Yoshimoto T. Cytokine frontiers: regulation of immune responses in health and disease. Springer;2013. DOI: 10.1007/978-4-431-54442-5.22. Kim H, Jang M, Kim Y, Choi J, Jeon J, Kim J, Hwang YI, Kang JS, Lee WJ. 2016; Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J Pharm Pharmacol. 68:406–20. DOI: 10.1111/jphp.12529. PMID: 26898166.23. Kim JH, Kim DH, Jo S, Cho MJ, Cho YR, Lee YJ, Byun S. 2022; Immunomodulatory functional foods and their molecular mechanisms. Exp Mol Med. 54:1–11. DOI: 10.1038/s12276-022-00724-0. PMID: 35079119. PMCID: PMC8787967.24. Jeong YJ, Kim JH, Hong JM, Kang JS, Kim HR, Lee WJ, Hwang YI. 2014; Vitamin C treatment of mouse bone marrow-derived dendritic cells enhanced CD8(+) memory T cell production capacity of these cells in vivo. Immunobiology. 219:554–64. DOI: 10.1016/j.imbio.2014.03.006. PMID: 24698552.25. Grudzien M, Rapak A. 2018; Effect of natural compounds on NK cell activation. J Immunol Res. 2018:4868417. DOI: 10.1155/2018/4868417. PMID: 30671486. PMCID: PMC6323526.26. Toliopoulos I, Simos Y, Verginadis I, Oikonomidis S, Karkabounas S. 2012; NK cell stimulation by administration of vitamin C and Aloe vera juice in vitro and in vivo: a pilot study. J Herb Med. 2:29–33. DOI: 10.1016/j.hermed.2012.04.002.27. Huijskens MJ, Walczak M, Sarkar S, Atrafi F, Senden-Gijsbers BL, Tilanus MG, Bos GM, Wieten L, Germeraad WT. 2015; Ascorbic acid promotes proliferation of natural killer cell populations in culture systems applicable for natural killer cell therapy. Cytotherapy. 17:613–20. DOI: 10.1016/j.jcyt.2015.01.004. PMID: 25747742.28. Mandl J, Szarka A, Bánhegyi G. 2009; Vitamin C: update on physiology and pharmacology. Br J Pharmacol. 157:1097–110. DOI: 10.1111/j.1476-5381.2009.00282.x. PMID: 19508394. PMCID: PMC2743829.29. Carr AC, Maggini S. 2017; Vitamin C and immune function. Nutrients. 9:1211. DOI: 10.3390/nu9111211. PMID: 29099763. PMCID: PMC5707683.30. Jacob RA, Sotoudeh G. 2002; Vitamin C function and status in chronic disease. Nutr Clin Care. 5:66–74. DOI: 10.1046/j.1523-5408.2002.00005.x. PMID: 12134712.31. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. 1981; Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 78:2879–82. DOI: 10.1073/pnas.78.5.2879. PMID: 6265920. PMCID: PMC319462.32. Stone N, Meister A. 1962; Function of ascorbic acid in the conversion of proline to collagen hydroxyproline. Nature. 194:555–7. DOI: 10.1038/194555a0. PMID: 13917472.33. Davey MW, Montagu MV, Inzé D, Sanmartin M, Kanellis A, Smirnoff N, Benzie IJ, Strain JJ, Favell D, Fletcher J. 2000; Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric. 80:825–60. DOI: 10.1002/(SICI)1097-0010(20000515)80:7<825::AID-JSFA598>3.0.CO;2-6.34. Kim Y, Kim H, Bae S, Choi J, Lim SY, Lee N, Kong JM, Hwang YI, Kang JS, Lee WJ. 2013; Vitamin C Is an essential factor on the anti-viral immune responses through the production of interferon-α/β at the initial stage of influenza A virus (H3N2) infection. Immune Netw. 13:70–4. DOI: 10.4110/in.2013.13.2.70. PMID: 23700397. PMCID: PMC3659258.35. Jo H, Lee D, Go C, Jang Y, Chu N, Bae S, Kang D, Im JP, Kim Y, Kang JS. 2022; Preventive effect of vitamin C on dextran sulfate sodium (DSS)-induced colitis via the regulation of IL-22 and IL-6 production in Gulo(-/-) mice. Int J Mol Sci. 23:10612. DOI: 10.3390/ijms231810612. PMID: 36142515. PMCID: PMC9505994.36. Boretti A, Banik BK. 2020; Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 12:100190. DOI: 10.1016/j.phanu.2020.100190. PMID: 32322486. PMCID: PMC7172861.37. Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. 1999; Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 17:189–220. DOI: 10.1146/annurev.immunol.17.1.189. PMID: 10358757.38. Bald T, Krummel MF, Smyth MJ, Barry KC. 2020; The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat Immunol. 21:835–47. DOI: 10.1038/s41590-020-0728-z. PMID: 32690952. PMCID: PMC8406687.39. Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. 2015; NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 6:368. DOI: 10.3389/fimmu.2015.00368. PMID: 26284063. PMCID: PMC4515552.40. Poggi A, Zocchi MR. 2014; NK cell autoreactivity and autoimmune diseases. Front Immunol. 5:27. DOI: 10.3389/fimmu.2014.00027. PMID: 24550913. PMCID: PMC3912987.41. Röthlisberger P, Hollenstein M. 2018; Aptamer chemistry. Adv Drug Deliv Rev. 134:3–21. DOI: 10.1016/j.addr.2018.04.007. PMID: 29626546.42. Wu Z, Tang LJ, Zhang XB, Jiang JH, Tan W. 2011; Aptamer-modified nanodrug delivery systems. ACS Nano. 5:7696–9. DOI: 10.1021/nn2037384. PMID: 22023403. PMCID: PMC3245875.43. Iliuk AB, Hu L, Tao WA. 2011; Aptamer in bioanalytical applications. Anal Chem. 83:4440–52. DOI: 10.1021/ac201057w. PMID: 21524128. PMCID: PMC3115435.44. Song KM, Lee S, Ban C. 2012; Aptamers and their biological applications. Sensors (Basel). 12:612–31. DOI: 10.3390/s120100612. PMID: 22368488. PMCID: PMC3279232.45. Kalathingal M, Rhee YM. 2023; Molecular mechanism of binding between a therapeutic RNA aptamer and its protein target VEGF: a molecular dynamics study. J Comput Chem. 44:1129–37. DOI: 10.1002/jcc.27070. PMID: 36625560.46. Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR. 2004; Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 351:2805–16. DOI: 10.1056/NEJMoa042760. PMID: 15625332.47. Ng EW, Shima DT, Calias P, Cunningham ET Jr, Guyer DR, Adamis AP. 2006; Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 5:123–32. DOI: 10.1038/nrd1955. PMID: 16518379.48. Kim H, Bae S, Yu Y, Kim Y, Kim HR, Hwang YI, Kang JS, Lee WJ. 2012; The analysis of vitamin C concentration in organs of gulo(-/-) mice upon vitamin C withdrawal. Immune Netw. 12:18–26. DOI: 10.4110/in.2012.12.1.18. PMID: 22536166. PMCID: PMC3329599.49. Jung SA, Lee DH, Moon JH, Hong SW, Shin JS, Hwang IY, Shin YJ, Kim JH, Gong EY, Kim SM, Lee EY, Lee S, Kim JE, Kim KP, Hong YS, Lee JS, Jin DH, Kim T, Lee WJ. 2016; L-ascorbic acid can abrogate SVCT-2-dependent cetuximab resistance mediated by mutant KRAS in human colon cancer cells. Free Radic Biol Med. 95:200–8. DOI: 10.1016/j.freeradbiomed.2016.03.009. PMID: 27012422.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-tumor Activity of Schoenoplectus triqueter Extract by Suppressing the STAT3 Signaling Pathway in A549 Lung Adenocarcinoma Cells
- Withaferin-A Inhibits Colon Cancer Cell Growth by Blocking STAT3 Transcriptional Activity
- Resveratrol from Peanut Sprout Extract Promotes NK Cell Activation and Antitumor Activity
- MiR-506 Promotes Natural Killer Cell Cytotoxicity against Human Hepatocellular Carcinoma Cells by Targeting STAT3
- Unraveling Stereochemical Structure-Activity Relationships of Sesquiterpene Lactones for Inhibitory Effects on STAT3 Activation