Anat Cell Biol.
2024 Sep;57(3):400-407. 10.5115/acb.23.313.
Alcohol intake during pregnancy reduces offspring bone epiphyseal growth plate chondrocyte proliferation through transforming growth factor β-1 inhibition in the Sprague Dawley rat humerus
- Affiliations
-
- 1Department of Human Anatomy and Histology, School of Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
- KMID: 2559758
- DOI: http://doi.org/10.5115/acb.23.313
Abstract
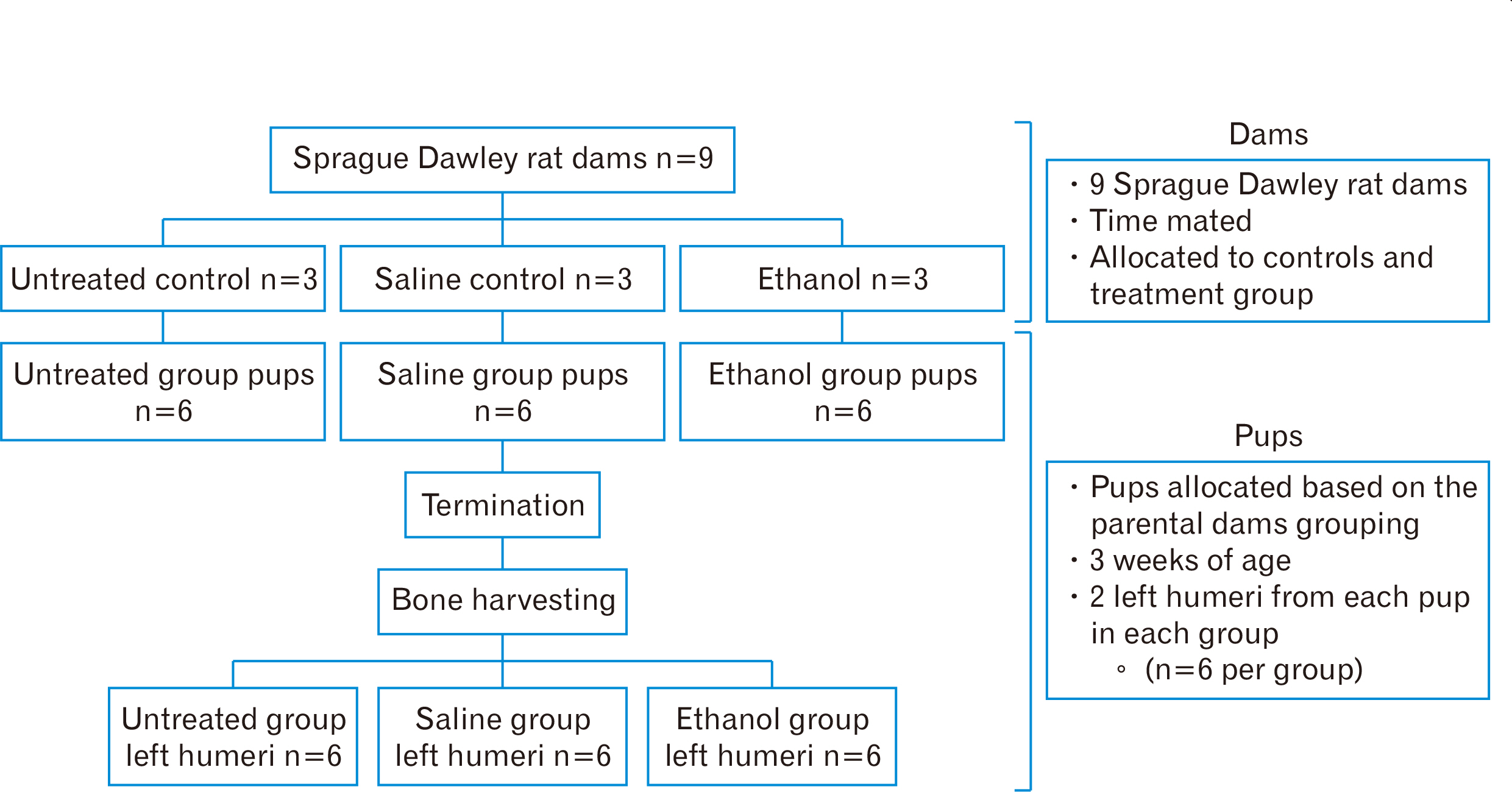
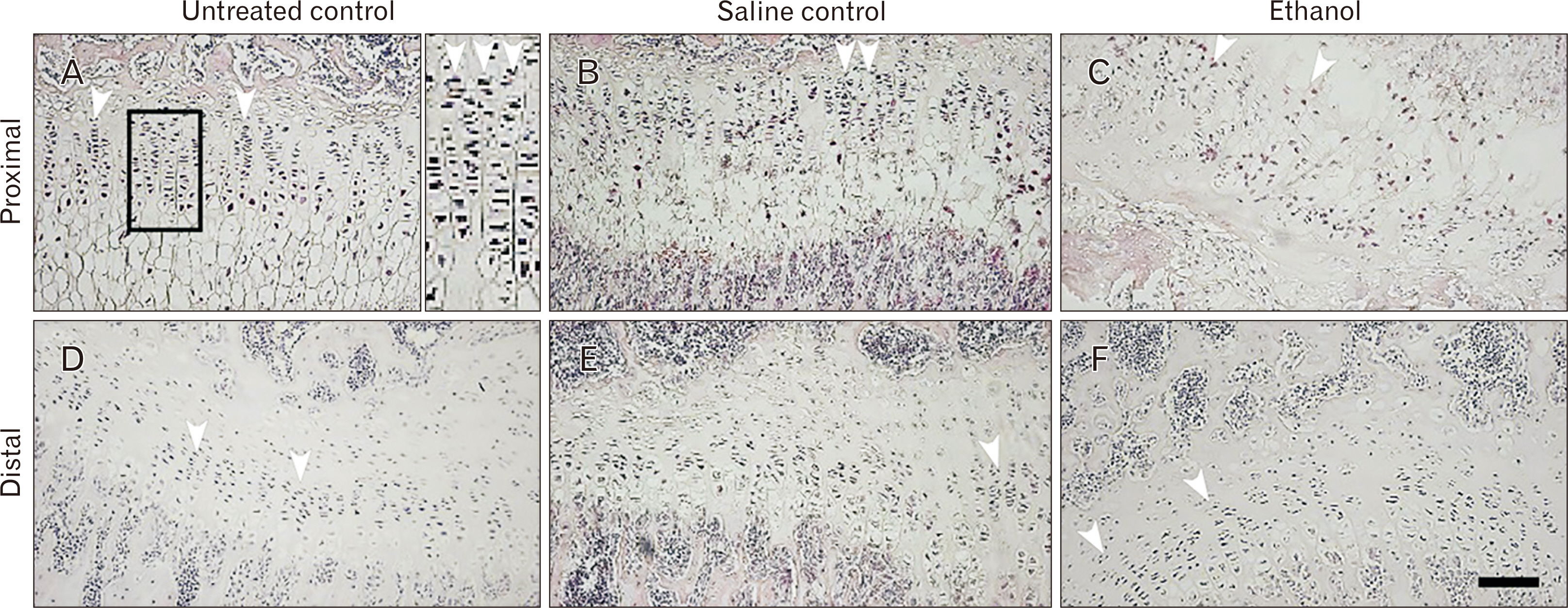
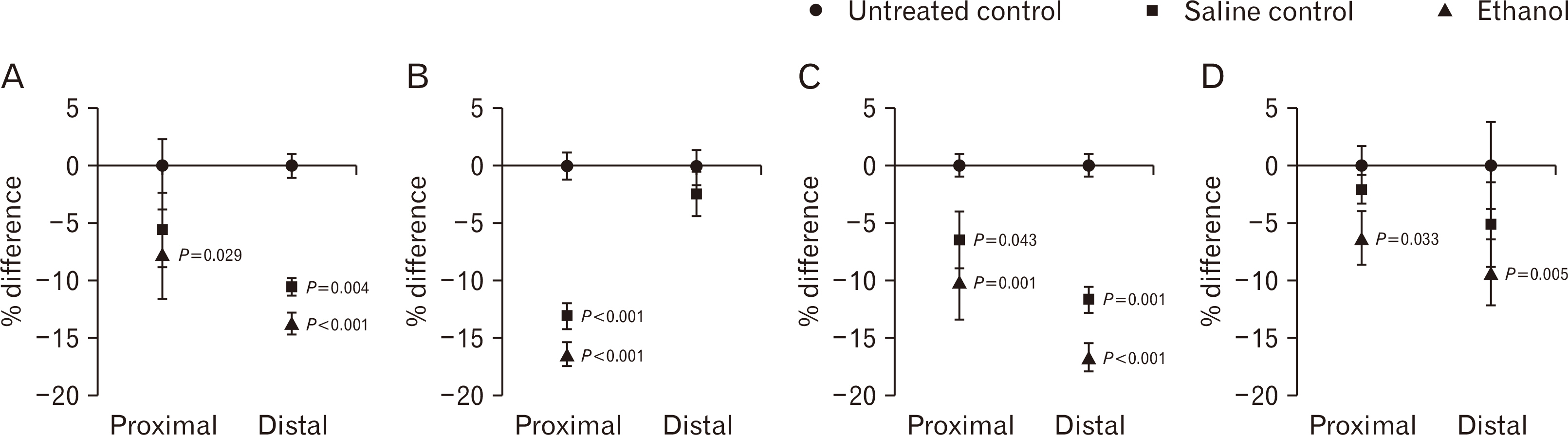
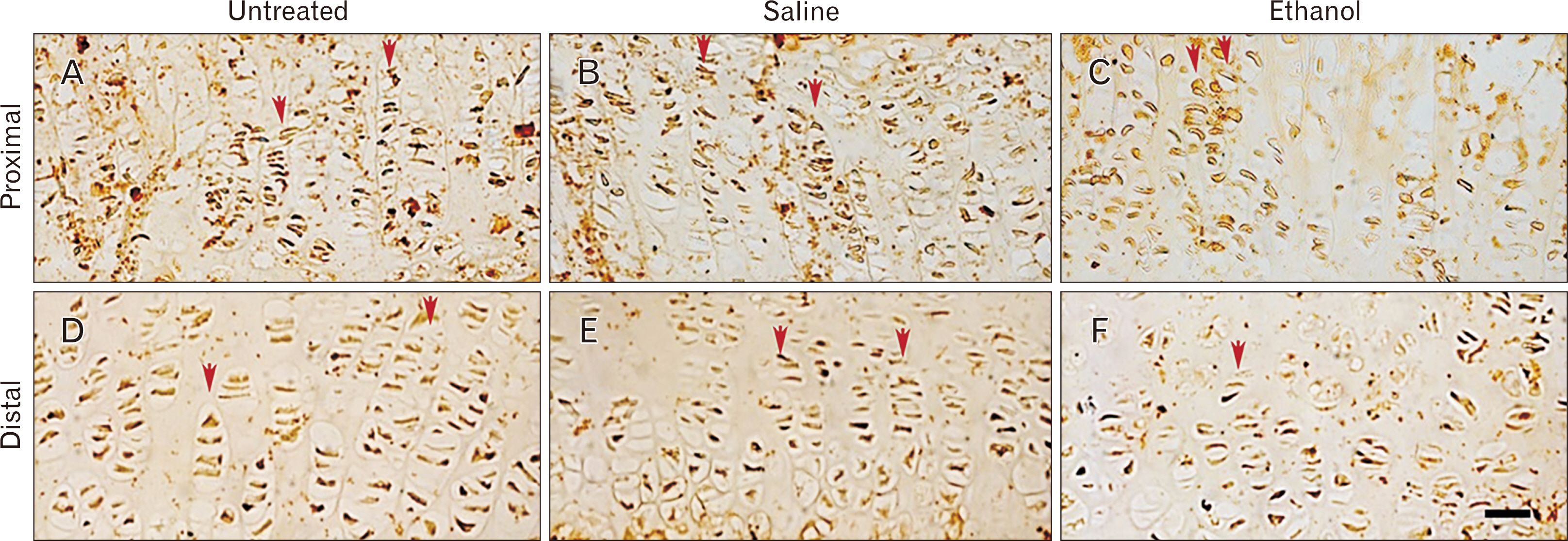
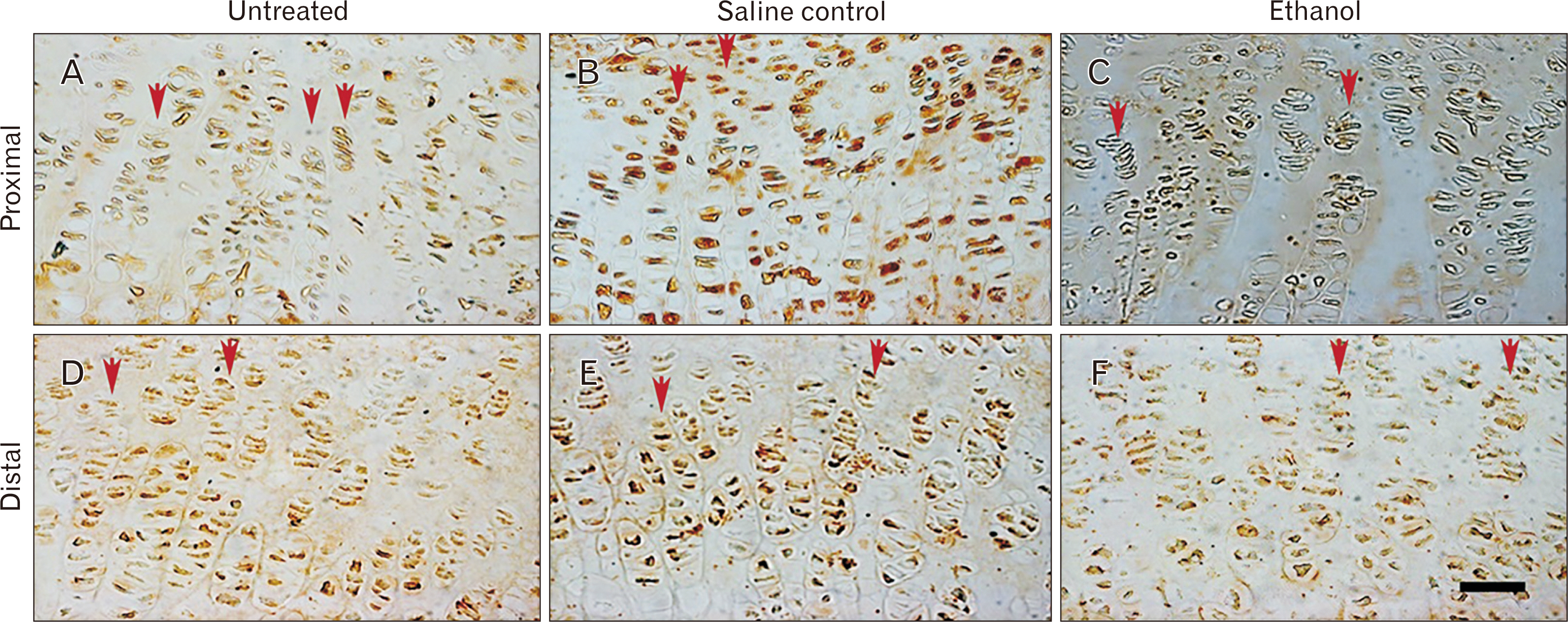
- Intrauterine alcohol exposure delays bone maturation and intensifies osteoporosis and fracture risk. As most studies emphasize the neurological aspects of intrauterine alcohol exposure, there is a lack of research on the implications pertaining to osseous tissue. Previous studies investigated these effects in fetuses, with limited studies on postnatal life. Postnatal studies are crucial since peak bone growth occurs during adolescence. This study aimed at assessing the effects of prenatal alcohol exposure on the humerus proximal and distal growth plate chondrocytes in 3-week-old rats. Sprague Dawley rats (n=9) were assigned to either the ethanol group (n=3), saline (n=3), and untreated (n=3) group and time-mated. Once pregnant, as confirmed by the presence of a copulation plug, the former 2 groups were treated with 0.015 ml/g of 25.2% ethanol and 0.9% saline. The untreated group received no treatment. The left humeri belonging to 6 pups per group were used. Serial sections were cut with a microtome at 5 µm thickness. These sections were stained with haematoxylin and eosin for assessment of normal morphology or immunolabeled with anti-Ki-67 and transforming growth factor β-1 (TGFβ-1) antibody. Prenatal alcohol exposure adversely effected the growth plate sizes and the number of cells in the proliferative zone. Fewer TGFβ-1 immunopositive and proliferative chondrocytes were found using the anti-Ki-67 antibody. This may explain the growth retardation in offspring exposed to gestational alcohol, showing that gestational alcohol exposure inhibits cell proliferation, aiding the diminished stature.
Figure
Reference
-
References
1. Pielage M, El Marroun H, Odendaal HJ, Willemsen SP, Hillegers MHJ, Steegers EAP, Rousian M. 2023; Alcohol exposure before and during pregnancy is associated with reduced fetal growth: the Safe Passage Study. BMC Med. 21:318. DOI: 10.1186/s12916-023-03020-4. PMID: 37612658. PMCID: PMC10463675.2. Simpson M, Duggal S, Keiver K. 2005; Prenatal ethanol exposure has differential effects on fetal growth and skeletal ossification. Bone. 36:521–32. DOI: 10.1016/j.bone.2004.11.011. PMID: 15777686.3. Snow ME, Keiver K. 2007; Prenatal ethanol exposure disrupts the histological stages of fetal bone development. Bone. 41:181–7. DOI: 10.1016/j.bone.2007.04.182. PMID: 17532282. PMCID: PMC2039868.4. Abel EL, Dintcheff BA. 1978; Effects of prenatal alcohol exposure on growth and development in rats. J Pharmacol Exp Ther. 207:916–21. PMID: 731439.5. Chaudhuri JD. 2000; Alcohol and the developing fetus--a review. Med Sci Monit. 6:1031–41. PMID: 11208451.6. Day NL, Jasperse D, Richardson G, Robles N, Sambamoorthi U, Taylor P, Scher M, Stoffer D, Cornelius M. 1989; Prenatal exposure to alcohol: effect on infant growth and morphologic characteristics. Pediatrics. 84:536–41. DOI: 10.1542/peds.84.3.536. PMID: 2771556.7. Miralles-Flores C, Delgado-Baeza E. 1992; Histomorphometric analysis of the epiphyseal growth plate in rats after prenatal alcohol exposure. J Orthop Res. 10:325–36. DOI: 10.1002/jor.1100100304. PMID: 1569495.8. Kanaan RA, Kanaan LA. 2006; Transforming growth factor beta1, bone connection. Med Sci Monit. 12:RA164–9. PMID: 16865078.9. Bismar H, Klöppinger T, Schuster EM, Balbach S, Diel I, Ziegler R, Pfeilschifter J. 1999; Transforming growth factor beta (TGF-beta) levels in the conditioned media of human bone cells: relationship to donor age, bone volume, and concentration of TGF-beta in human bone matrix in vivo. Bone. 24:565–9. DOI: 10.1016/S8756-3282(99)00082-4. PMID: 10375198.10. Kumar M, Dandapat S, Sinha MP, Kumar A, Raipat BS. 2017; Different blood collection methods from rats: a review. Balneo Res J. 8:46–50. DOI: 10.12680/balneo.2017.141.11. Parasuraman S, Raveendran R, Kesavan R. 2010; Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 1:87–93. Erratum in: J Pharmacol Pharmacother 2017;8: 153. DOI: 10.4103/0976-500X.72350. PMID: 29081629. PMCID: PMC5642135.12. Tiscione NB, Alford I, Yeatman DT, Shan X. 2011; Ethanol analysis by headspace gas chromatography with simultaneous flame-ionization and mass spectrometry detection. J Anal Toxicol. 35:501–11. DOI: 10.1093/anatox/35.7.501. PMID: 21871160.13. Balcombe JP, Barnard ND, Sandusky C. 2004; Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 43:42–51. PMID: 15669134.14. Kelly RR, McDonald LT, Jensen NR, Sidles SJ, LaRue AC. 2019; Impacts of psychological stress on osteoporosis: clinical implications and treatment interactions. Front Psychiatry. 10:200. DOI: 10.3389/fpsyt.2019.00200. PMID: 31024360. PMCID: PMC6465575.15. Chen G, Deng C, Li YP. 2012; TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 8:272–88. DOI: 10.7150/ijbs.2929. PMID: 22298955. PMCID: PMC3269610.16. Pillay DS, Ndou R. 2022; Intrauterine alcohol exposure delays growth and disturbs trabecular morphology in 3-week-old Sprague - Dawley rat femur. J Anat Soc India. 71:93–101. DOI: 10.4103/jasi.jasi_183_21.17. Dlamini GF, Ndou R. 2023; Osteoblastogenesis and osteolysis in the Zucker Diabetic Sprague Dawley rat humerus head. Anat Cell Biol. 56:552–61. DOI: 10.5115/acb.23.166. PMID: 37788886. PMCID: PMC10714090.18. Pillay D, Ndou R. 2021; Intrauterine alcohol exposure disturbs trabecular morphology in the Sprague Dawley rat humerus epiphysis up to 3-weeks postnatally. Int J Morphol. 39:1436–42. DOI: 10.4067/S0717-95022021000501436.19. Ndou R, Bello NK, Perry V, Pillay D. 2023; Distal tibial trabecular morphometry in a Sprague Dawley rat model of fetal alcohol syndrome: a micro focus X-ray computed tomography case-control study. Pan Afr Med J. 46:35. DOI: 10.11604/pamj.2023.46.35.37151. PMID: 38145202. PMCID: PMC10746879.20. Nwaogu IC. 2002; Foetal alcohol syndrome: growth rate of bones in rats. J Appl Anim Res. 22:249–53. DOI: 10.1080/09712119.2002.9706406.21. Janssens K, ten Dijke P, Janssens S, Van Hul W. 2005; Transforming growth factor-beta1 to the bone. Endocr Rev. 26:743–74. DOI: 10.1210/er.2004-0001. PMID: 15901668.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pubertal growth and epiphyseal fusion
- Transplantation of Growth Plate Chondrocytes into Growth Plate Defect in Rabbit
- Growth plate closure and therapeutic interventions
- Proximal Humeral Fracture with Epiphyseal Plate Injury
- Molecular mechanisms of long bone growth and chondrocyte regulation: A narrative review