Korean J Pain.
2024 Oct;37(4):320-331. 10.3344/kjp.24196.
Down-regulation of microRNA-382-5p reduces neuropathic pain by targeting regulation of dual specificity phosphatase-1
- Affiliations
-
- 1Department of Pain Management, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Department of Anaesthesia and Surgery, Hebei Maternity Hospital, Shijiazhuang, China
- 3Department of Anesthesiology and Surgery, Shengli Oilfield Central Hospital, Dongying, China
- 4Department of Pain, Shengli Oilfield Central Hospital, Dongying, China
- 5Shandong Second Medical University, Weifang, China
- 6Department of Anesthesiology, Dongying Hospital of Traditional Chinese Medicine, Dongying, China
- 7Department of Anesthesiology, The First Hospital of China Medical University, Shenyang, China
- KMID: 2559694
- DOI: http://doi.org/10.3344/kjp.24196
Abstract
- Background
MicroRNA (miRNA) plays a crucial role in neuropathic pain (NP) by targeting mRNAs. This study aims to analyze the regulatory function and mechanism of miR-382-5p/dual specificity phosphatase-1 (DUSP1) axis in NP.
Methods
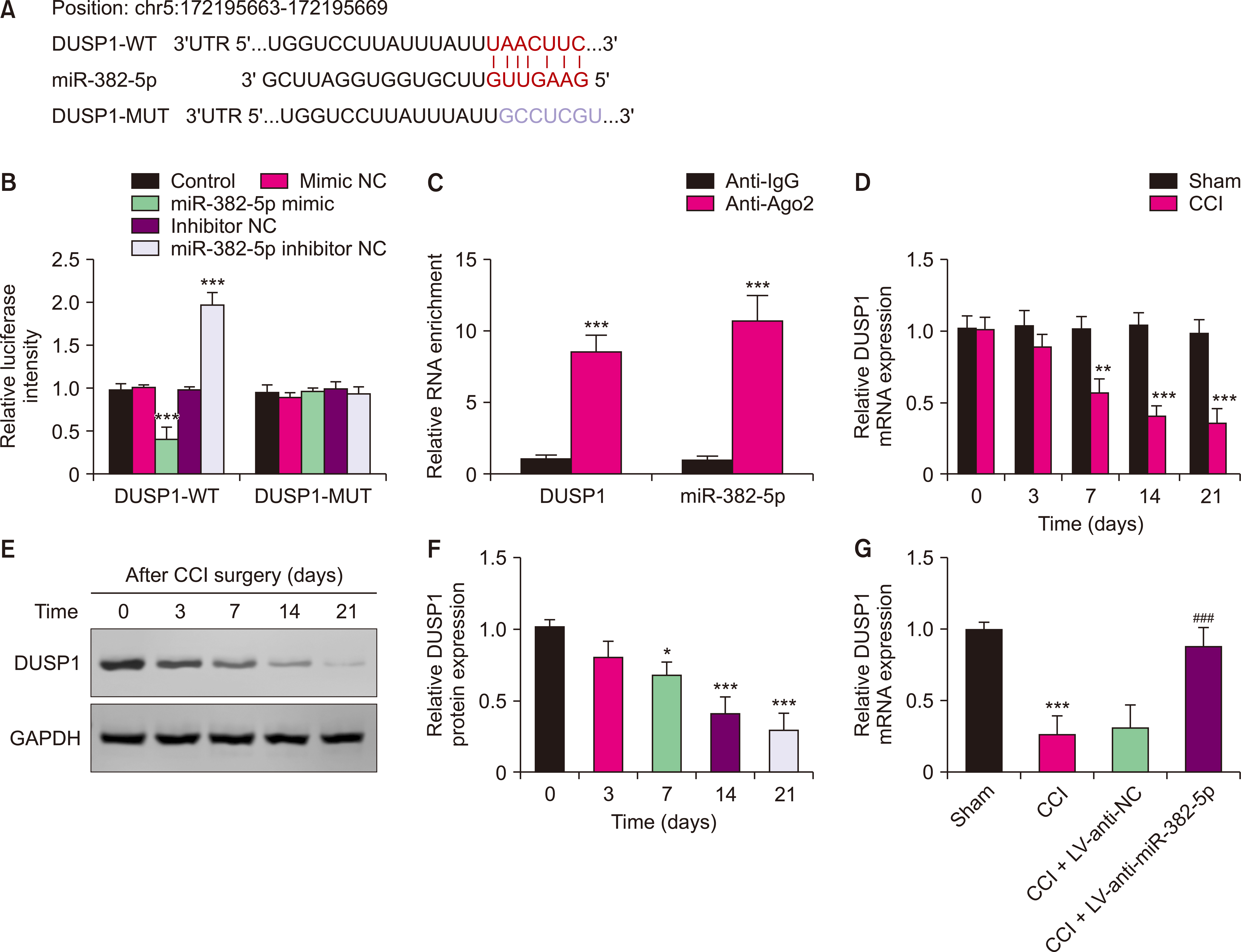
We utilized rats with chronic constriction injury (CCI) of the sciatic nerve as the NP model. The levels of miR-382-5p and DUSP1 were reduced by intrathecal injection of lentiviral interference vectors targeting miR-382-5p and DUSP1. The mRNA levels of miR-382-5p and DUSP1 in the dorsal root ganglions (DRGs) were measured by RT-qPCR assay. The pain behavior was evaluated by mechanical nociceptive sensitivity and thermal nociceptive sensitivity. The expression levels of interleukin-6 (IL)-6, IL-1β, and tumor necrosis factor-α in the DRGs were analyzed by ELISA assay. The targeting relationship between miR-382-5p and DUSP1 was verified by DLR assay and RIP assay.
Results
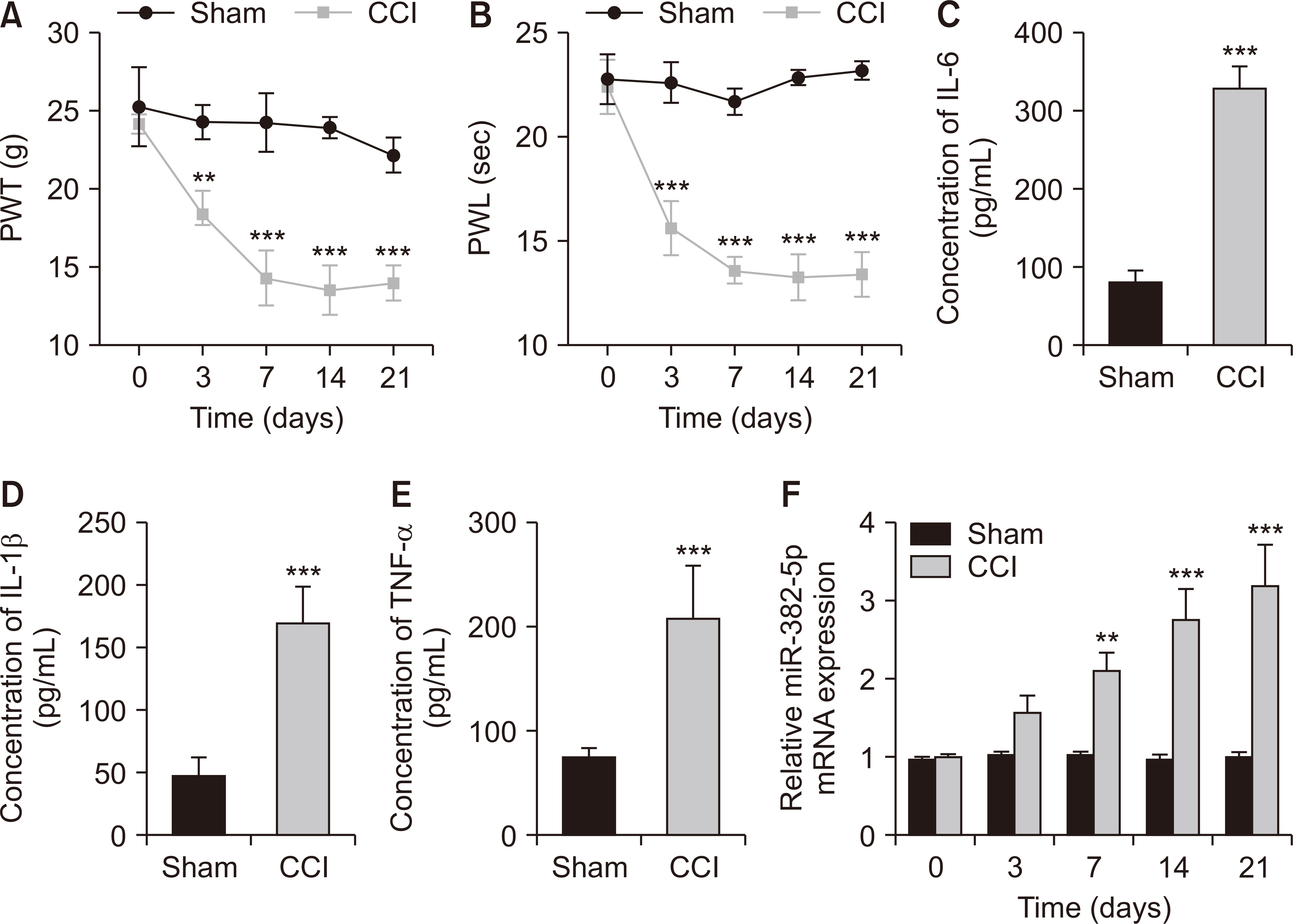
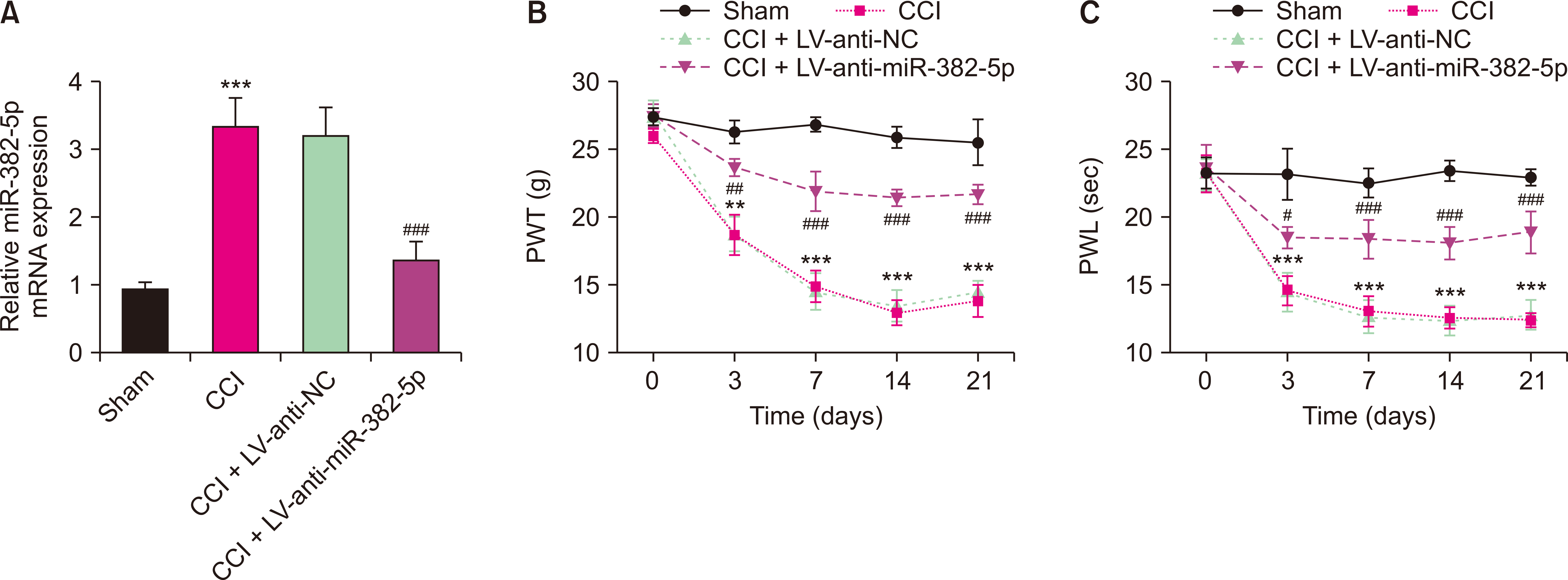
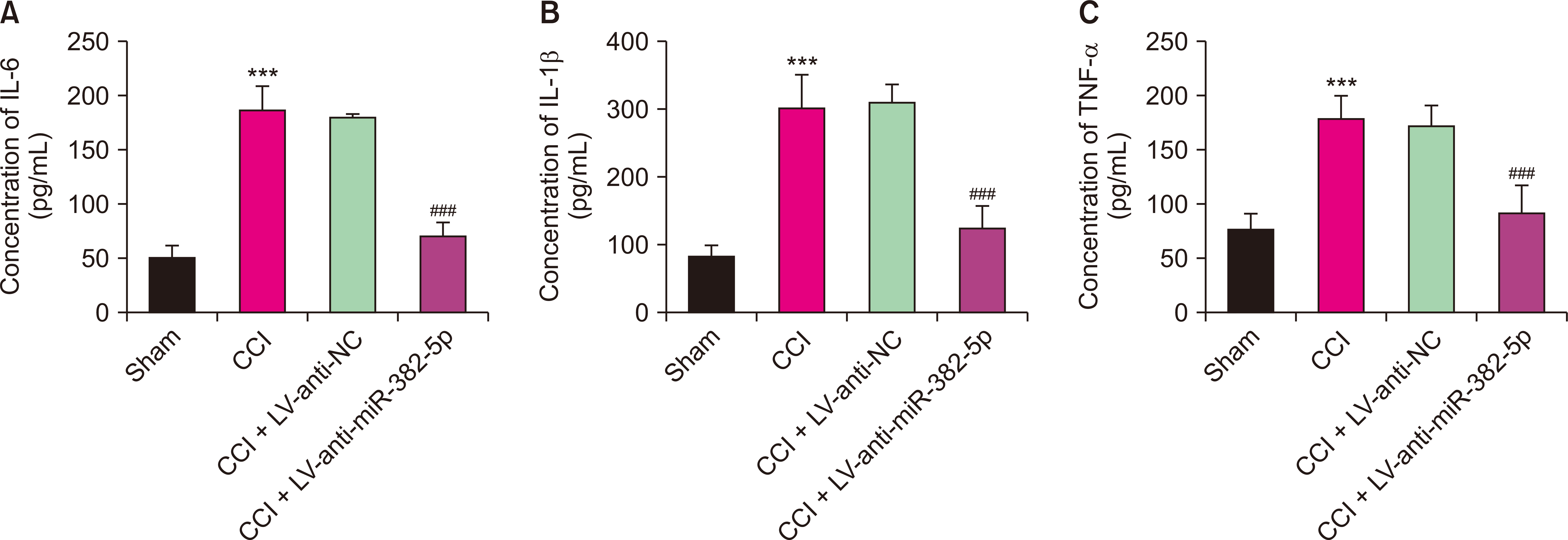
Compared to the Sham group, the CCI rats exhibited higher levels of miR-382-5p and lower levels of DUSP1. Overexpression of miR-382-5p significantly decreased DUSP1 levels. Reducing miR-382-5p levels can lower the mechanical nociceptive sensitivity and thermal nociceptive sensitivity of CCI rats and inhibit the over-activation of pro-inflammatory factors. Reduced miR-382-5p levels decreased NP in CCI rats. DUSP1 is the target of miR-382-5p, and down-regulation of DUSP1 reverses the inhibitory effect of reduced miR-382-5p levels on NP.
Conclusions
Down-regulation of miR-382-5p inhibits the over-activation of pro-inflammatory factors by targeting and regulating the expression of DUPS1, thereby alleviating NP.
Keyword
Figure
Reference
-
1. Moini Zanjani T, Ameli H, Labibi F, Sedaghat K, Sabetkasaei M. 2014; The attenuation of pain behavior and serum COX-2 concentration by curcumin in a rat model of neuropathic pain. Korean J Pain. 27:246–52. DOI: 10.3344/kjp.2014.27.3.246. PMID: 25031810. PMCID: PMC4099237.2. Gilron I, Baron R, Jensen T. 2015; Neuropathic pain: principles of diagnosis and treatment. Mayo Clin Proc. 90:532–45. DOI: 10.1016/j.mayocp.2015.01.018. PMID: 25841257.3. Reisig F, Harnik M. 2020; [Neuropathic pain: pharmacotherapy]. Ther Umsch. 77:274–280. German.DOI: 10.1024/0040-5930/a001191. PMID: 32930074.4. Elzahaf RA, Johnson MI, Tashani OA. 2016; The epidemiology of chronic pain in Libya: a cross-sectional telephone survey. BMC Public Health. 16:776. DOI: 10.1186/s12889-016-3349-6. PMID: 27514513. PMCID: PMC4982430.5. Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. 2017; Neuropathic pain. Nat Rev Dis Primers. 3:17002. DOI: 10.1038/nrdp.2017.2. PMID: 28205574. PMCID: PMC5371025.6. Friedman RC, Farh KK, Burge CB, Bartel DP. 2009; Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19:92–105. DOI: 10.1101/gr.082701.108. PMID: 18955434. PMCID: PMC2612969.7. López-González MJ, Landry M, Favereaux A. 2017; MicroRNA and chronic pain: from mechanisms to therapeutic potential. Pharmacol Ther. 180:1–15. DOI: 10.1016/j.pharmthera.2017.06.001. PMID: 28579386.8. Xiang W, Jiang L, Zhou Y, Li Z, Zhao Q, Wu T, et al. 2021; The lncRNA Ftx/miR-382-5p/Nrg1 axis improves the inflammation response of microglia and spinal cord injury repair. Neurochem Int. 143:104929. DOI: 10.1016/j.neuint.2020.104929. PMID: 33359189.9. Yin X, Zheng W, He L, Mu S, Shen Y, Wang J. 2022; CircHIPK3 alleviates inflammatory response and neuronal apoptosis via regulating miR-382-5p/DUSP1 axis in spinal cord injury. Transpl Immunol. 73:101612. DOI: 10.1016/j.trim.2022.101612. PMID: 35500847.10. Ashmwe M, Posa K, Rührnößl A, Heinzel JC, Heimel P, Mock M, et al. 2022; Effects of extracorporeal shockwave therapy on functional recovery and circulating miR-375 and miR-382-5p after subacute and chronic spinal cord contusion injury in rats. Biomedicines. 10:1630. DOI: 10.3390/biomedicines10071630. PMID: 35884935. PMCID: PMC9313454.11. Cao S, Zhang D, Yuan J, Liu C, Zhou W, Zhang L, et al. 2019; MicroRNA and circular RNA expression in affected skin of patients with postherpetic neuralgia. J Pain Res. 12:2905–13. DOI: 10.2147/JPR.S221615. PMID: 31695480. PMCID: PMC6802488.12. Wang X, Jiang Y, Li J, Wang Y, Tian Y, Guo Q, et al. 2021; DUSP1 promotes microglial polarization toward M2 phenotype in the medial prefrontal cortex of neuropathic pain rats via inhibition of MAPK pathway. ACS Chem Neurosci. 12:966–78. DOI: 10.1021/acschemneuro.0c00567. PMID: 33666084.13. Liu LP, Zhang J, Pu B, Li WQ, Wang YS. 2020; Upregulation of JHDM1D-AS1 alleviates neuroinflammation and neuronal injury via targeting miR-101-3p-DUSP1 in spinal cord after brachial plexus injury. Int Immunopharmacol. 89:106962. DOI: 10.1016/j.intimp.2020.106962. PMID: 33039970.14. Li Z, Li A, Yan L, Yang T, Xu W, Fan P. 2020; Downregulation of long noncoding RNA DLEU1 attenuates hypersensitivity in chronic constriction injury-induced neuropathic pain in rats by targeting miR-133a-3p/SRPK1 axis. Mol Med. 26:104. DOI: 10.1186/s10020-020-00235-6. PMID: 33167866. PMCID: PMC7653812.15. Yoo SH, Lee SH, Lee S, Park JH, Lee S, Jin H, et al. 2020; The effect of human mesenchymal stem cell injection on pain behavior in chronic post-ischemia pain mice. Korean J Pain. 33:23–9. DOI: 10.3344/kjp.2020.33.1.23. PMID: 31888314. PMCID: PMC6944374.16. Bennett GJ, Xie YK. 1988; A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 33:87–107. DOI: 10.1016/0304-3959(88)90209-6. PMID: 2837713.17. Attal N, Jazat F, Kayser V, Guilbaud G. 1990; Further evidence for 'pain-related' behaviours in a model of unilateral peripheral mononeuropathy. Pain. 41:235–51. DOI: 10.1016/0304-3959(90)90022-6.18. Bakare AO, Owoyele BV. 2020; Antinociceptive and neuroprotective effects of bromelain in chronic constriction injury-induced neuropathic pain in Wistar rats. Korean J Pain. 33:13–22. DOI: 10.3344/kjp.2020.33.1.13. PMID: 31888313. PMCID: PMC6944371.19. Pasquinelli AE. 2012; MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. 13:271–82. DOI: 10.1038/nrg3162. PMID: 22411466.20. Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. 2019; An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 234:5451–65. DOI: 10.1002/jcp.27486. PMID: 30471116.21. Kovanur Sampath K, Belcher S, Hales J, Thomson OP, Farrell G, Gisselman AS, et al. 2023; The role of micro-RNAs in neuropathic pain-a scoping review. Pain Rep. 8:e1108. DOI: 10.1097/PR9.0000000000001108. PMID: 37928202. PMCID: PMC10624461.22. Liu JC, Xue DF, Wang XQ, Ai DB, Qin PJ. 2019; MiR-101 relates to chronic peripheral neuropathic pain through targeting KPNB1 and regulating NF-κB signaling. Kaohsiung J Med Sci. 35:139–45. DOI: 10.1002/kjm2.12025. PMID: 30887716.23. Zhang YU, Ye G, Zhao J, Chen Y, Kong L, Sheng C, et al. 2022; Exosomes carried miR-181c-5p alleviates neuropathic pain in CCI rat models. An Acad Bras Cienc. 94:e20210564. DOI: 10.1590/0001-3765202220210564. PMID: 35976364.24. Kuffler DP. 2020; Mechanisms for reducing neuropathic pain. Mol Neurobiol. 57:67–87. DOI: 10.1007/s12035-019-01757-9. PMID: 31813127.25. Woolf CJ. 2011; Central sensitization: implications for the diagnosis and treatment of pain. Pain. 152(3 Suppl):S2–15. DOI: 10.1016/j.pain.2010.09.030. PMID: 20961685. PMCID: PMC3268359.26. Calvo M, Dawes JM, Bennett DL. 2012; The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 11:629–42. DOI: 10.1016/S1474-4422(12)70134-5. PMID: 22710756.27. Taylor DM, Moser R, Régulier E, Breuillaud L, Dixon M, Beesen AA, et al. 2013; MAP kinase phosphatase 1 (MKP-1/DUSP1) is neuroprotective in Huntington's disease via additive effects of JNK and p38 inhibition. J Neurosci. 33:2313–25. DOI: 10.1523/JNEUROSCI.4965-11.2013. PMID: 23392662. PMCID: PMC3711389.28. Zhang CG, Wan HQ, Ma KN, Luan SX, Li H. 2019; Identification of biomarkers related to neuropathic pain induced by peripheral nerve injury. J Mol Neurosci. 69:505–15. DOI: 10.1007/s12031-019-01322-y. PMID: 31352588.29. Xuan C, Cui H, Jin Z, Yue Y, Cao S, Cui S, et al. 2023; Glutamine ameliorates hyperoxia-induced hippocampal damage by attenuating inflammation and apoptosis via the MKP-1/MAPK signaling pathway in neonatal rats. Front Pharmacol. 14:1096309. DOI: 10.3389/fphar.2023.1096309. PMID: 36817145. PMCID: PMC9932780.30. Fan M, Wang C, Zhao X, Jiang Y, Wang C. 2023; Parthenolide alleviates microglia-mediated neuroinflammation via MAPK/TRIM31/NLRP3 signaling to ameliorate cognitive disorder. Int Immunopharmacol. 120:110287. DOI: 10.1016/j.intimp.2023.110287. PMID: 37182449.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MicroRNA-98-5p Inhibits Tumorigenesis of Hepatitis B Virus-Related Hepatocellular Carcinoma by Targeting NF-κB-Inducing Kinase
- MicroRNA profiling of tacrolimus-stimulated Jurkat human T lympocytes
- MicroRNA-139-5p Regulates Fibrotic Potentials via Modulation of Collagen Type 1 and Phosphorylated p38 MAPK in Uterine Leiomyoma
- Kartogenin Promotes the BMSCs Chondrogenic Differentiation in Osteoarthritis by Down-Regulation of miR-145-5p Targeting Smad4 Pathway
- Spinal Gap Junction Channels in Neuropathic Pain