Obstet Gynecol Sci.
2024 Sep;67(5):449-466. 10.5468/ogs.24120.
The evolving landscape of immunohistochemistry in cervical and uterine carcinoma in gynecologic oncology: current status and future directions
- Affiliations
-
- 1Department of Obstetrics & Gynaecology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India
- 2Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India
- 3Department of General Surgery, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India
- KMID: 2559477
- DOI: http://doi.org/10.5468/ogs.24120
Abstract
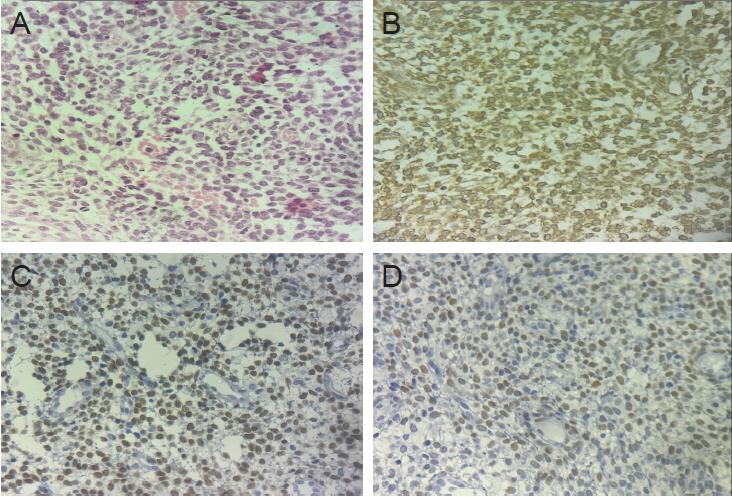
- Immunohistochemistry (IHC) has become an indispensable tool in routine gynecological pathology, particularly with the advancements in molecular understanding and histological classification of gynecological cancers. This evolution has led to new immunostainings for diagnostic and classification purposes. This review describes the diagnostic utility of IHC in gynecological neoplasms, drawing insights from literature reviews, personal experiences, and research findings. It delves into the application of IHC in resolving morphologically equivocal cases, emphasizing its role in achieving an accurate diagnosis. The selection of appropriate immunomarkers for common scenarios encountered in gynecological pathology aids pathologists in navigating complex cases. Specifically, we focus on cervical and endometrial malignancies, elucidating the molecular rationale behind the use of specific immunohistochemical markers. An updated overview of essential immunohistochemical markers provides knowledge for precise diagnosis and classification of gynecological cancers. This review serves as a valuable resource for clinicians and researchers involved in the management and study of gynecological malignancies, facilitating improved patient care and outcomes.
Keyword
Figure
Reference
-
References
1. Krishnamurti U, Movahedi-Lankarani S, Bell DA. Protocol for the examination of resection specimens from patients with primary carcinoma of the uterine cervix [Internet]. Chicago: College of American Pathologists;c2020. [cited 2024 Jan 11]. Available from: https://documents.cap.org/protocols/cp-gynecologic-uterinecervixresection-20-5000.pdf.2. Bhatla N, Berek JS, Cuello Fredes M, Denny LA, Grenman S, Karunaratne K, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019; 145:129–35.
Article3. Stolnicu S, Barsan I, Hoang L, Patel P, Terinte C, Pesci A, et al. International endocervical adenocarcinoma criteria and classification (IECC): a new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol. 2018; 42:214–26.4. Diaz De Vivar A, Roma AA, Park KJ, Alvarado-Cabrero I, Rasty G, Chanona-Vilchis JG, et al. Invasive endocervical adenocarcinoma: proposal for a new pattern-based classification system with significant clinical implications: a multi-institutional study. Int J Gynecol Pathol. 2013; 32:592–601.5. Yemelyanova A, Vang R, Seidman JD, Gravitt PE, Ronnett BM. Endocervical adenocarcinomas with prominent endometrial or endomyometrial involvement simulating primary endometrial carcinomas: utility of HPV DNA detection and immunohistochemical expression of p16 and hormone receptors to confirm the cervical origin of the corpus tumor. Am J Surg Pathol. 2009; 33:914–24.6. Chiesa-Vottero AG, Malpica A, Deavers MT, Broaddus R, Nuovo GJ, Silva EG. Immunohistochemical overexpression of p16 and p53 in uterine serous carcinoma and ovarian high-grade serous carcinoma. Int J Gynecol Pathol. 2007; 26:328–33.
Article7. McCluggage WG, Hurrell DP, Kennedy K. Metastatic carcinomas in the cervix mimicking primary cervical adenocarcinoma and adenocarcinoma in situ: report of a series of cases. Am J Surg Pathol. 2010; 34:735–41.8. Glenn McCluggage W. Immunohistochemistry as a diagnostic aid in cervical pathology. Pathology. 2007; 39:97–111.
Article9. McCluggage WG, Sumathi VP, McBride HA, Patterson A. A panel of immunohistochemical stains, including carcinoembryonic antigen, vimentin, and estrogen receptor, aids the distinction between primary endometrial and endocervical adenocarcinomas. Int J Gynecol Pathol. 2002; 21:11–5.10. Staebler A, Sherman ME, Zaino RJ, Ronnett BM. Hormone receptor immunohistochemistry and human papillomavirus in situ hybridization are useful for distinguishing endocervical and endometrial adenocarcinomas. Am J Surg Pathol. 2002; 26:998–1006.
Article11. Nofech-Mozes S, Khalifa MA, Ismiil N, Saad RS, Hanna WM, Covens A, et al. Immunophenotyping of serous carcinoma of the female genital tract. Mod Pathol. 2008; 21:1147–55.
Article12. Roma AA, Mistretta TA, Diaz De Vivar A, Park KJ, Alvarado-Cabrero I, Rasty G, et al. New pattern-based personalized risk stratification system for endocervical adenocarcinoma with important clinical implications and surgical outcome. Gynecol Oncol. 2016; 141:36–42.
Article13. Talia KL, McCluggage WG. The developing spectrum of gastric-type cervical glandular lesions. Pathology. 2018; 50:122–33.
Article14. Zhao S, Hayasaka T, Osakabe M, Kato N, Nakahara K, Kurachi H, et al. Mucin expression in nonneoplastic and neoplastic glandular epithelia of the uterine cervix. Int J Gynecol Pathol. 2003; 22:393–7.15. Lee S, Sahasrabuddhe VV, Mendoza-Cervantes D, Zhao R, Duggan MA. Tissue-based immunohistochemical biomarker expression in malignant glandular lesions of the uterine cervix: a systematic review. Int J Gynecol Pathol. 2018; 37:128–40.
Article16. Carleton C, Hoang L, Sah S, Kiyokawa T, Karamurzin YS, Talia KL, et al. A detailed immunohistochemical analysis of a large series of cervical and vaginal gastric-type adenocarcinomas. Am J Surg Pathol. 2016; 40:636–44.
Article17. Howitt BE, Emori MM, Drapkin R, Gaspar C, Barletta JA, Nucci MR, et al. GATA3 is a sensitive and specific marker of benign and malignant mesonephric lesions in the lower female genital tract. Am J Surg Pathol. 2015; 39:1411–9.
Article18. Roma AA, Goyal A, Yang B. Differential expression patterns of GATA3 in uterine mesonephric and nonmesonephric lesions. Int J Gynecol Pathol. 2015; 34:480–6.
Article19. Ju B, Wang J, Yang B, Sun L, Guo Y, Hao Q, et al. Morphologic and immunohistochemical study of clear cell carcinoma of the uterine endometrium and cervix in comparison to ovarian clear cell carcinoma. Int J Gynecol Pathol. 2018; 37:388–96.20. Spaans VM, Scheunhage DA, Barzaghi B, de Kroon CD, Fleuren GJ, Bosse T, et al. Independent validation of the prognostic significance of invasion patterns in endocervical adenocarcinoma: pattern a predicts excellent survival. Gynecol Oncol. 2018; 151:196–201.
Article21. Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018; 31:1770–86.
Article22. Howitt BE, Kelly P, McCluggage WG. Pathology of neuroendocrine tumours of the female genital tract. Curr Oncol Rep. 2017; 19:59.
Article23. Ganesan R, Hirschowitz L, Dawson P, Askew S, Pearmain P, Jones PW, et al. Neuroendocrine carcinoma of the cervix: review of a series of cases and correlation with outcome. Int J Surg Pathol. 2016; 24:490–6.24. Perunovic B, Sunassee A. Small cell neuroendocrine carcinoma [Internet]. Bingham Farms: PathologyOutlines;c2024. [cited 2024 Jan 24]. Available from: https://www.pathologyoutlines.com/topic/cervixsmallcell.html.25. Huang W, Liu J, Xu K, Chen H, Bian C. PD-1/PD-L1 inhibitors for advanced or metastatic cervical cancer: from bench to bed. Front Oncol. 2022; 12:849352.
Article26. Lantuejoul S, Sound-Tsao M, Cooper WA, Girard N, Hirsch FR, Roden AC, et al. PD-L1 testing for lung cancer in 2019: perspective from the IASLC pathology committee. J Thorac Oncol. 2020; 15:499–519.
Article27. Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020; 10:727–42.28. Ansari-Lari MA, Staebler A, Zaino RJ, Shah KV, Ronnett BM. Distinction of endocervical and endometrial adenocarcinomas: immunohistochemical p16 expression correlated with human papillomavirus (HPV) DNA detection. Am J Surg Pathol. 2004; 28:160–7.29. Kong CS, Beck AH, Longacre TA. A panel of 3 markers including p16, ProExC, or HPV ISH is optimal for distinguishing between primary endometrial and endocervical adenocarcinomas. Am J Surg Pathol. 2010; 34:915–26.30. Saad RS, Mashhour M, Noftech-Mozes S, Ismiil N, Dubé V, Ghorab Z, et al. P16INK4a expression in undifferentiated carcinoma of the uterus does not exclude its endometrial origin. Int J Gynecol Pathol. 2012; 31:57–65.
Article31. McCluggage WG, Soslow RA, Gilks CB. Patterns of p53 immunoreactivity in endometrial carcinomas: ‘all or nothing’ staining is of importance. Histopathology. 2011; 59:786–8.
Article32. Houghton O, McCluggage WG. The expression and diagnostic utility of p63 in the female genital tract. Adv Anat Pathol. 2009; 16:316–21.
Article33. Bartosch C, Manuel Lopes J, Oliva E. Endometrial carcinomas: a review emphasizing overlapping and distinctive morphological and immunohistochemical features. Adv Anat Pathol. 2011; 18:415–37.34. Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol. 2013; 37:874–81.35. Laury AR, Perets R, Piao H, Krane JF, Barletta JA, French C, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011; 35:816–26.
Article36. Ordóñez NG. Value of PAX 8 immunostaining in tumor diagnosis: a review and update. Adv Anat Pathol. 2012; 19:140–51.37. Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008; 32:1566–71.
Article38. Shukla A, Thomas D, Roh MH. PAX8 and PAX2 expression in endocervical adenocarcinoma in situ and high-grade squamous dysplasia. Int J Gynecol Pathol. 2013; 32:116–21.
Article39. Köbel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG. Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol. 2019; 38 Suppl 1:S123–31.40. Baniak N, Fadare O, Köbel M, DeCoteau J, Parkash V, Hecht JL, et al. Targeted molecular and immunohistochemical analyses of endometrial clear cell carcinoma show that POLE mutations and DNA mismatch repair protein deficiencies are uncommon. Am J Surg Pathol. 2019; 43:531–37.
Article41. Chen W, Husain A, Nelson GS, Rambau PF, Liu S, Lee CH, et al. Immunohistochemical profiling of endometrial serous carcinoma. Int J Gynecol Pathol. 2017; 36:128–39.
Article42. Kommoss F, Faruqi A, Gilks CB, Lamshang Leen S, Singh N, Wilkinson N, et al. Uterine serous carcinomas frequently metastasize to the fallopian tube and can mimic serous tubal intraepithelial carcinoma. Am J Surg Pathol. 2017; 41:161–70.
Article43. McNamara B, Mutlu L, Greenman M, Harold J, Santin A. HER2 oncogene as molecular target in uterine serous carcinoma and uterine carcinosarcoma. Cancers (Basel). 2023; 15:4085.
Article44. Glasspool RM, McNeish IA. Clear cell carcinoma of ovary and uterus. Curr Oncol Rep. 2013; 15:566–72.45. Fadare O, Liang SX. Diagnostic utility of hepatocyte nuclear factor 1-beta immunoreactivity in endometrial carcinomas: lack of specificity for endometrial clear cell carcinoma. Appl Immunohistochem Mol Morphol. 2012; 20:580–7.46. Fadare O, Zhao C, Khabele D, Parkash V, Quick CM, Gwin K, et al. Comparative analysis of Napsin A, alphamethylacyl-coenzyme A racemase (AMACR, P504S), and hepatocyte nuclear factor 1 beta as diagnostic markers of ovarian clear cell carcinoma: an immunohistochemical study of 279 ovarian tumours. Pathology. 2015; 47:105–11.
Article47. Hariri N, Qarmali M, Fadare O. Endometrial serous carcinoma with clear-cell change: frequency and immunohistochemical analysis. Int J Surg Pathol. 2018; 26:126–34.
Article48. Li Z, Zhao C. Clinicopathologic and immunohistochemical characterization of dedifferentiated endometrioid adenocarcinoma. Appl Immunohistochem Mol Morphol. 2016; 24:562–8.
Article49. Tafe LJ, Garg K, Chew I, Tornos C, Soslow RA. Endometrial and ovarian carcinomas with undifferentiated components: clinically aggressive and frequently underrecognized neoplasms. Mod Pathol. 2010; 23:781–9.50. Shah VI, Ramalingam P, McCluggage WG. CD34 expression in undifferentiated endometrial carcinoma. Histopathology. 2016; 69:894–7.
Article51. Ramalingam P, Croce S, McCluggage WG. Loss of expression of SMARCA4 (BRG1), SMARCA2 (BRM) and SMARCB1 (INI1) in undifferentiated carcinoma of the endometrium is not uncommon and is not always associated with rhabdoid morphology. Histopathology. 2017; 70:359–66.
Article52. Tipps AM, Plaxe SC, Weidner N. Endometrioid carcinoma with a low-grade spindle cell component: a tumor resembling an adnexal tumor of probable Wolffian origin. Ann Diagn Pathol. 2011; 15:376–81.
Article53. Stewart AP. Genetic testing strategies in newly diagnosed endometrial cancer patients aimed at reducing morbidity or mortality from lynch syndrome in the index case or her relatives. PLoS Curr. 2013; 5:ecurrents.eogt.b59a6e84f27c536e50db4e46aa26309c.
Article54. Watkins JC, Nucci MR, Ritterhouse LL, Howitt BE, Sholl LM. Unusual mismatch repair immunohistochemical patterns in endometrial carcinoma. Am J Surg Pathol. 2016; 40:909–16.55. Pai RK, Plesec TP, Abdul-Karim FW, Yang B, Marquard J, Shadrach B, et al. Abrupt loss of MLH1 and PMS2 expression in endometrial carcinoma: molecular and morphologic analysis of 6 cases. Am J Surg Pathol. 2015; 39:993–9.56. van den Heerik ASVM, Horeweg N, de Boer SM, Bosse T, Creutzberg CL. Adjuvant therapy for endometrial cancer in the era of molecular classification: radiotherapy, chemoradiation and novel targets for therapy. Int J Gynecol Cancer. 2021; 31:594–604.
Article57. Ogunmuyiwa J, Williams V. Emerging advances in endometrial cancer: integration of molecular classification into staging for enhanced prognostic accuracy and implications for racial disparities. Cancers (Basel). 2024; 16:1172.
Article58. Oliva E. Practical issues in uterine pathology from banal to bewildering: the remarkable spectrum of smooth muscle neoplasia. Mod Pathol. 2016; 29 Suppl 1:S104–20.
Article59. Zhang Q, Kanis MJ, Ubago J, Liu D, Scholtens DM, Strohl AE, et al. The selected biomarker analysis in 5 types of uterine smooth muscle tumors. Hum Pathol. 2018; 76:17–27.60. Hakverdi S, Güngören A, Yaldiz M, Hakverdi AU, Toprak S. Immunohistochemical analysis of p16 expression in uterine smooth muscle tumors. Eur J Gynaecol Oncol. 2011; 32:513–5.61. Hoang LN, Aneja A, Conlon N, Delair DF, Middha S, Benayed R, et al. Novel high-grade endometrial stromal sarcoma: a morphologic mimicker of myxoid leiomyosarcoma. Am J Surg Pathol. 2017; 41:12–24.62. Parra-Herran C, Schoolmeester JK, Yuan L, Dal Cin P, Fletcher CD, Quade BJ, et al. Myxoid leiomyosarcoma of the uterus: a clinicopathologic analysis of 30 cases and review of the literature with reappraisal of its distinction from other uterine myxoid mesenchymal neoplasms. Am J Surg Pathol. 2016; 40:285–301.63. Parra-Herran C, Quick CM, Howitt BE, Dal Cin P, Quade BJ, Nucci MR. Inflammatory myofibroblastic tumor of the uterus: clinical and pathologic review of 10 cases including a subset with aggressive clinical course. Am J Surg Pathol. 2015; 39:157–68.64. O’Neill CJ, McBride HA, Connolly LE, McCluggage WG. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology. 2007; 50:851–8.65. Hwang H, Matsuo K, Duncan K, Pakzamir E, Pham HQ, Correa A, et al. Immunohistochemical panel to differentiate endometrial stromal sarcoma, uterine leiomyosarcoma and leiomyoma: something old and something new. J Clin Pathol. 2015; 68:710–7.
Article66. Micci F, Gorunova L, Agostini A, Johannessen LE, Brunetti M, Davidson B, et al. Cytogenetic and molecular profile of endometrial stromal sarcoma. Genes Chromosomes Cancer. 2016; 55:834–46.
Article67. Isphording A, Ali RH, Irving J, Goytain A, Nelnyk N, Hoang LN, et al. YWHAE-FAM22 endometrial stromal sarcoma: diagnosis by reverse transcription-polymerase chain reaction in formalin-fixed, paraffin-embedded tumor. Hum Pathol. 2013; 44:837–43.
Article68. Lee CH, Mariño-Enriquez A, Ou W, Zhu M, Ali RH, Chiang S, et al. The clinicopathologic features of YWHAEFAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012; 36:641–53.69. Conlon N, Soslow RA, Murali R. Perivascular epithelioid tumours (PEComas) of the gynaecological tract. J Clin Pathol. 2015; 68:418–26.70. Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005; 29:1558–75.71. Rao Q, Cheng L, Xia QY, Liu B, Li L, Shi QL, et al. Cathepsin K expression in a wide spectrum of perivascular epithelioid cell neoplasms (PEComas): a clinicopathological study emphasizing extrarenal PEComas. Histopathology. 2013; 62:642–50.
Article72. Gulavita P, Fletcher CDM, Hirsch MS. PNL2: an adjunctive biomarker for renal angiomyolipomas and perivascular epithelioid cell tumours. Histopathology. 2018; 72:441–8.
Article73. Bennett JA, Nardi V, Rouzbahman M, Morales-Oyarvide V, Nielsen GP, Oliva E. Inflammatory myofibroblastic tumor of the uterus: a clinicopathological, immunohistochemical, and molecular analysis of 13 cases highlighting their broad morphologic spectrum. Mod Pathol. 2017; 30:1489–503.
Article74. Haimes JD, Stewart CJR, Kudlow BA, Culver BP, Meng B, Koay E, et al. Uterine inflammatory myofibroblastic tumors frequently harbor ALK fusions with IGFBP5 and THBS1. Am J Surg Pathol. 2017; 41:773–80.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The role of human papillomavirus testing after treatment for high-grade cervical dysplasia
- Current status and future directions of ovarian cancer prognostic models
- Expression of Fragile Histidine Triad (FHIT) Gene Product in the Uterine Cervical Carcinoma
- Who should be offered non-radical surgery for early-stage cervical cancer?
- Gynecologic oncology in 2024: breakthrough trials and evolving treatment strategies for cervical, uterine corpus, and ovarian cancers