Obstet Gynecol Sci.
2024 Sep;67(5):435-448. 10.5468/ogs.22084.
Genetic landscape of thrombophilia in recurrent miscarriages
- Affiliations
-
- 1Department of Biosciences, Jamia Millia Islamia, New Delhi, India
- 2Department of Obstetrics and Gynaecology, Maulana Azad Medical College, New Delhi, India
- 3Department of Pathology, Era’s Medical College, Lucknow, India
- KMID: 2559476
- DOI: http://doi.org/10.5468/ogs.22084
Abstract
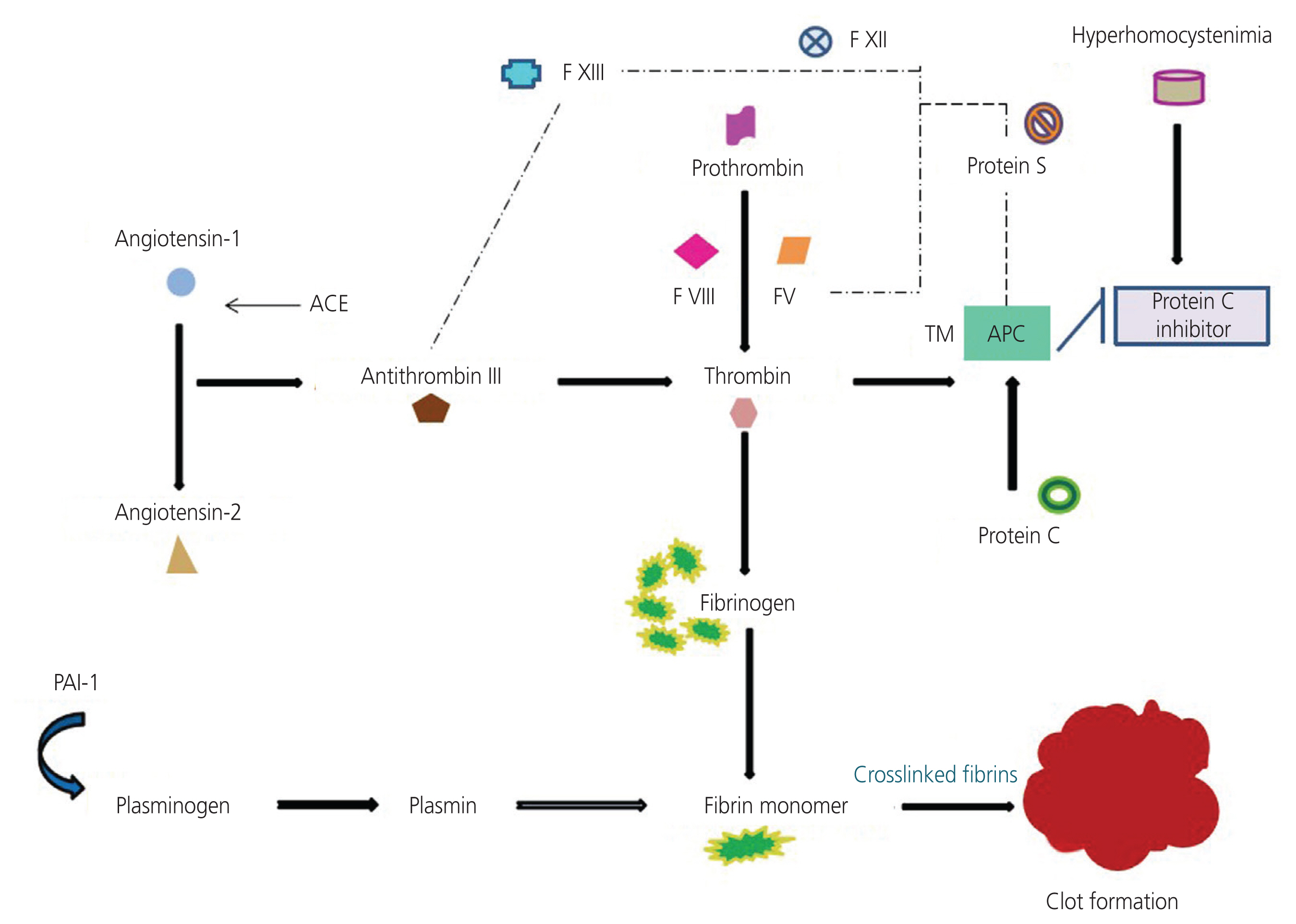
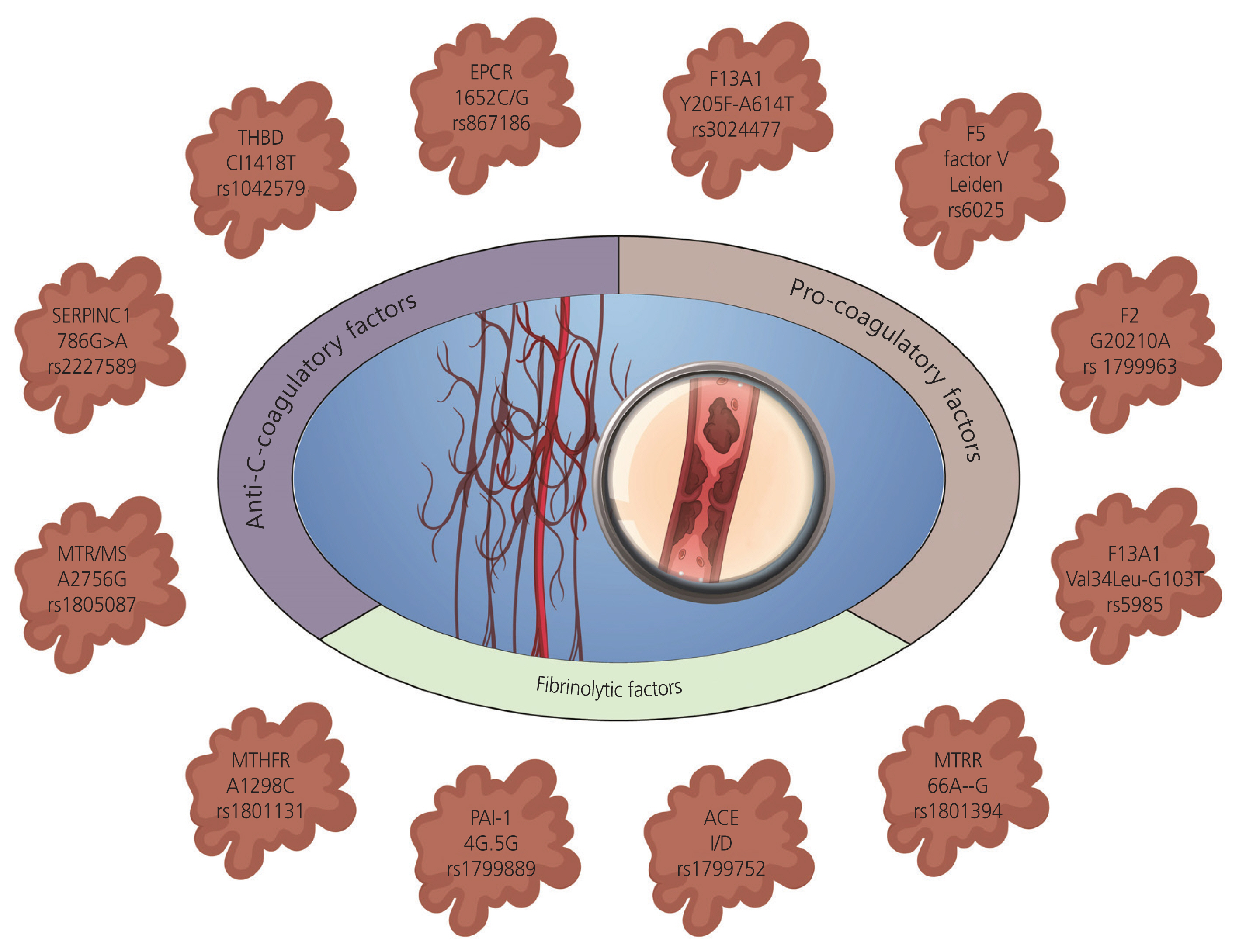
- The etiology of recurrent miscarriage (RM) is extremely heterogeneous, encompassing genetic, immunological, anatomical, endocrine, thrombophilic, infectious, and uterine abnormalities. Thrombophilia is a major contributor to pregnancy complications, potentially harming the fetus and jeopardizing the continuation of pregnancy. Therefore, successful pregnancy outcomes depend on maintaining a delicate balance between coagulation and fibrinolytic factors, crucial for ensuring the adjustment of the basal plate to facilitate adequate placental perfusion. Despite numerous studies shedding light on the role of thrombophilic factors and genetic variations in RM, the exact pathogenesis remains unclear. It is imperative to systematically rule out thrombophilia and other related factors responsible for pregnancy disorders and RMs to guide appropriate and active management strategies. Addressing thrombophilia continues to present challenges in terms of effective treatment. The current review aims to address the heterogeneity of RM as a therapeutic challenge, emphasizing the need for standardized diagnostic tests and welldesigned multicenter research trials to gather robust, evidence-based data on thrombophilic causes of RM and provide effective treatment. The goal is to enhance the understanding of thrombophilic factors and genetic landscapes associated with RM through various approaches, including candidate gene studies, genome-wide association studies, and high-throughput sequencing. Meta-analyses have underscored the significance of genetic aberrations in RM, highlighting the necessity for identifying critical mutations implicated in the etiopathogenesis of miscarriages to pave the way for implementation of targeted clinical therapies.
Keyword
Figure
Reference
-
References
1. Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018; 2018:hoy004.2. Hyde KJ, Schust DJ. Genetic considerations in recurrent pregnancy loss. Cold Spring Harb Perspect Med. 2015; 5:a023119.3. Shah K, Bhat P, Bhat R, Sultana R. An update on recurrent early pregnancy loss: causes, controversies and cure. J Clin Diagn Res. 2018; 12:1–5.
Article4. Wang NF, Kolte AM, Larsen EC, Nielsen HS, Christiansen OB. Immunologic abnormalities, treatments, and recurrent pregnancy loss: what is real and what is not? Clin Obstet Gynecol. 2016; 59:509–23.
Article5. Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009; 2:76–83.6. Walker ID. Thrombophilia in pregnancy. J Clin Pathol. 2000; 53:573–80.
Article7. De Santis M, Cavaliere AF, Straface G, Di Gianantonio E, Caruso A. Inherited and acquired thrombophilia: pregnancy outcome and treatment. Reprod Toxicol. 2006; 22:227–33.8. Divya Pandey D, Gupta S. Current update on recurrent pregnancy loss. J Basic Clin Reprod Sci. 2019; 8:1–6.9. Coriu L, Ungureanu R, Talmaci R, Uscatescu V, Cirstoiu M, Coriu D, et al. Hereditary thrombophilia and thrombotic events in pregnancy: single-center experience. J Med Life. 2014; 7:567–71.10. Jadaon MM. Epidemiology of activated protein C resistance and factor V leiden mutation in the mediterranean region. Mediterr J Hematol Infect Dis. 2011; 3:e2011037.
Article11. Buchholz T, Thaler CJ. Inherited thrombophilia: impact on human reproduction. Am J Reprod Immunol. 2003; 50:20–32.
Article12. Murin S, Marelich GP, Arroliga AC, Matthay RA. Hereditary thrombophilia and venous thromboembolism. Am J Respir Crit Care Med. 1998; 158:1369–73.13. Corral J, Roldán V, Vicente V. Deep venous thrombosis or pulmonary embolism and factor V Leiden: enigma or paradox. Haematologica. 2010; 95:863–6.
Article14. El Hachem H, Crepaux V, May-Panloup P, Descamps P, Legendre G, Bouet PE. Recurrent pregnancy loss: current perspectives. Int J Womens Health. 2017; 9:331–45.
Article15. Nazari L, Salehpour S, Hosseini S, Hashemi T, Borumandnia N, Azizi E. Effect of autologous platelet-rich plasma for treatment of recurrent pregnancy loss: a randomized controlled trial. Obstet Gynecol Sci. 2022; 65:266–72.
Article16. Torabi R, Zarei S, Zeraati H, Zarnani AH, Akhondi MM, Hadavi R, et al. Combination of thrombophilic gene polymorphisms as a cause of increased the risk of recurrent pregnancy loss. J Reprod Infertil. 2012; 13:89–94.17. Coulam CB, Jeyendran RS, Fishel LA, Roussev R. Multiple thrombophilic gene mutations rather than specific gene mutations are risk factors for recurrent miscarriage. Am J Reprod Immunol. 2006; 55:360–8.18. Mishra P, Singh K, Tyagi S, Juneja R, Mahapatra M. Inherited and acquired thrombophilia in women of Indian ethnicity with recurrent pregnancy loss: an observational study from North India. Indian J Pathol Microbiol. 2021; 64:741–5.19. Sedano-Balbás S, Lyons M, Cleary B, Murray M, Gaffney G, Maher M. Acquired activated protein C resistance, thrombophilia and adverse pregnancy outcomes: a study performed in an Irish cohort of pregnant women. J Pregnancy. 2011; 2011:232840.
Article20. McNamee K, Dawood F, Farquharson R. Recurrent miscarriage and thrombophilia: an update. Curr Opin Obstet Gynecol. 2012; 24:229–34.21. Eslami MM, Khalili M, Soufizomorrod M, Abroun S, Razi B. Factor V Leiden 1691G > a mutation and the risk of recurrent pregnancy loss (RPL): systematic review and meta-analysis. Thromb J. 2020; 18:11.
Article22. Parveen F, Shukla A, Agrawal S. Should factor V Leiden mutation and prothrombin gene polymorphism testing be done in women with recurrent miscarriage from North India? Arch Gynecol Obstet. 2013; 287:375–81.23. Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and fibrin in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2017; 37:e13–21.
Article24. Renné T, Schmaier AH, Nickel KF, Blombäck M, Maas C. In vivo roles of factor XII. Blood. 2012; 120:4296–303.
Article25. Jyotsna PL, Sharma S, Trivedi SS. Coagulation inhibitors and activated protein C resistance in recurrent pregnancy losses in Indian women. Indian J Pathol Microbiol. 2011; 54:752–5.
Article26. Aiach M, Borgel D, Gaussem P, Emmerich J, Alhenc-Gelas M, Gandrille S. Protein C and protein S deficiencies. Semin Hematol. 1997; 34:205–16.27. Soare AM, Popa C. Deficiencies of proteins C, S and antithrombin and activated protein C resistance--their involvement in the occurrence of arterial thromboses. J Med Life. 2010; 3:412–5.28. Mekaj Y, Lulaj S, Daci F, Rafuna N, Miftari E, Hoxha H, et al. Prevalence and role of antithrombin III, protein C and protein S deficiencies and activated protein C resistance in Kosovo women with recurrent pregnancy loss during the first trimester of pregnancy. J Hum Reprod Sci. 2015; 8:224–9.
Article29. Sabadell J, Casellas M, Alijotas-Reig J, Arellano-Rodrigo E, Cabero L. Inherited antithrombin deficiency and pregnancy: maternal and fetal outcomes. Eur J Obstet Gynecol Reprod Biol. 2010; 149:47–51.
Article30. Tait RC, Walker ID, Perry DJ, Islam SI, Daly ME, McCall F, et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol. 1994; 87:106–12.
Article31. Bogdanova N, Markoff A. Hereditary thrombophilic risk factors for recurrent pregnancy loss. J Community Genet. 2010; 1:47–53.
Article32. Barut MU, Bozkurt M, Kahraman M, Yıldırım E, Imirzalioğlu N, Kubar A, et al. Thrombophilia and recurrent pregnancy loss: the enigma continues. Med Sci Monit. 2018; 24:4288–94.33. Ye Y, Vattai A, Zhang X, Zhu J, Thaler CJ, Mahner S, et al. Role of plasminogen activator inhibitor type 1 in pathologies of female reproductive diseases. Int J Mol Sci. 2017; 18:1651.
Article34. Yang C, Fangfang W, Jie L, Yanlong Y, Jie W, Xuefei L, et al. Angiotensin-converting enzyme insertion/deletion (I/D) polymorphisms and recurrent pregnancy loss: a meta-analysis. J Assist Reprod Genet. 2012; 29:1167–73.
Article35. Parveen F, Tripathi G, Singh B, Faridi RM, Agrawal S. Acetylcholinesterase gene polymorphism and recurrent pregnancy loss. Int J Gynaecol Obstet. 2009; 106:68–9.
Article36. Ozgu-Erdinc AS, Togrul C, Aktulay A, Buyukkagnici U, Yapar Eyi EG, Erkaya S. Factor XII (Hageman) levels in women with recurrent pregnancy loss. J Pregnancy. 2014; 2014:459192.
Article37. Parveen F, Tuteja M, Agrawal S. Polymorphisms in MTH-FR, MTHFD, and PAI-1 and recurrent miscarriage among North Indian women. Arch Gynecol Obstet. 2013; 288:1171–7.38. Saraswathy KN, Kaur L, Talwar S, Mishra J, Huidrom S, Sachdeva MP, et al. Methylenetetrahydrofolate reductase gene-specific methylation and recurrent miscarriages: a case-control study from North India. J Hum Reprod Sci. 2018; 11:142–7.
Article39. Pandey K, Dubay P, Bhagoliwal A, Gupta N, Tyagi G. Hyperhomocysteinemia as a risk factor for IUGR. J Obstet Gynaecol India. 2012; 62:406–8.
Article40. Raziel A, Kornberg Y, Friedler S, Schachter M, Sela BA, Ron-El R. Hypercoagulable thrombophilic defects and hyperhomocysteinemia in patients with recurrent pregnancy loss. Am J Reprod Immunol. 2001; 45:65–71.
Article41. Ivanov P, Tsvyatkovska T, Konova E, Komsa-Penkova R. Inherited thrombophilia and IVF failure: the impact of coagulation disorders on implantation process. Am J Reprod Immunol. 2012; 68:189–98.42. Grandone E, Piazza G. Thrombophilia, inflammation, and recurrent pregnancy loss: a case-based review. Semin Reprod Med. 2021; 39:62–8.43. Parveen F, Shukla A, Agarwal S. Cytokine gene polymorphisms in northern Indian women with recurrent miscarriages. Fertil Steril. 2013; 99:433–40.44. Wawrusiewicz-Kurylonek N, Krętowski AJ, Posmyk R. Frequency of thrombophilia associated genes variants: population-based study. BMC Med Genet. 2020; 9(21):198.
Article45. Pereza N, Ostojić S, Kapović M, Peterlin B. Systematic review and meta-analysis of genetic association studies in idiopathic recurrent spontaneous abortion. Fertil Steril. 2017; 107:150–9e2.
Article46. Parveen F, Agrawal S. A study of forty-seven single nucleotide polymorphisms among recurrent miscarriage using classification and regression tree analysis. Am J Reprod Immunol. 2013; 70:529–37.
Article47. Rull K, Nagirnaja L, Laan M. Genetics of recurrent miscarriage: challenges, current knowledge, future directions. Front Genet. 2012; 3:34.48. Loizidou EM, Kucherenko A, Tatarskyy P, Chernushyn S, Livshyts G, Gulkovskyi R, et al. Risk of recurrent pregnancy loss in the ukrainian population using a combined effect of genetic variants: a case-control study. Genes (Basel). 2021; 12:64.
Article49. Kim MS, Gu BH, Song S, Choi BC, Cha DH, Baek KH. ITI-H4, as a biomarker in the serum of recurrent pregnancy loss (RPL) patients. Mol Biosyst. 2011; 7:1430–40.
Article50. Laisk T, Soares ALG, Ferreira T, Painter JN, Censin JC, Laber S, et al. The genetic architecture of sporadic and multiple consecutive miscarriage. Nat Commun. 2020; 11:5980.
Article51. Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011; 12:745–55.
Article52. Quintero-Ronderos P, Mercier E, Fukuda M, González R, Suárez CF, Patarroyo MA, et al. Novel genes and mutations in patients affected by recurrent pregnancy loss. PLoS One. 2017; 12:e0186149.53. de Ligt J, Veltman JA, Vissers LE. Point mutations as a source of de novo genetic disease. Curr Opin Genet Dev. 2013; 23:257–63.
Article54. Lindström S, Wang L, Smith EN, Gordon W, van Hylckama Vlieg A, de Andrade M, et al. Genomic and transcriptomic association studies identify 16 novel susceptibility loci for venous thromboembolism. Blood. 2019; 134:1645–57.
Article55. Klarin D, Busenkell E, Judy R, Lynch J, Levin M, Haessler J, et al. Genome-wide association analysis of venous thromboembolism identifies new risk loci and genetic overlap with arterial vascular disease. Nat Genet. 2019; 51:1574–9.
Article56. Merriman L, Greaves M. Testing for thrombophilia: an evidence-based approach. Postgrad Med J. 2006; 82:699–704.
Article57. Lockwood CJ. Inherited thrombophilias in pregnant patients: detection and treatment paradigm. Obstet Gynecol. 2002; 99:333–41.58. Ortel TL. Antiphospholipid syndrome: laboratory testing and diagnostic strategies. Am J Hematol. 2012; 87(Suppl 1):S75–81.
Article59. Silva M, de Leeuw N, Mann K, Schuring-Blom H, Morgan S, Giardino D, et al. European guidelines for constitutional cytogenomic analysis. Eur J Hum Genet. 2019; 27:1–16.
Article60. Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009; 60:39–54.
Article61. Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004; 10:1222–6.
Article62. Ahn TG, Hwang JY. Preeclampsia and aspirin. Obstet Gynecol Sci. 2023; 66:120–32.63. Kim YM, Sung JH, Cha HH, Oh SY. Hydroxychloroquine in obstetrics: potential implications of the prophylactic use of hydroxychloroquine for placental insufficiency during pregnancy. Obstet Gynecol Sci. 2024; 67:143–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recurrent pregnancy loss: can factor V Leiden mutations be a cause
- Inherited thrombophilia profile in patients with recurrent miscarriages: Experience from a tertiary care center in north India
- A De Novo Centric Fission of Chromosome 11 in a Patient with Recurrent Miscarriages
- Evaluation of Chromosomal Analyses done on the Parents with Chromosomal Anomalous Children and Recurrent Abortion
- First Korean case of factor V Leiden mutation in pregnant woman with a history of recurrent pregnancy loss