J Liver Cancer.
2024 Sep;24(2):192-205. 10.17998/jlc.2024.04.05.
Inter-reader agreement for CT/MRI LI-RADS category M imaging features: a systematic review and meta-analysis
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2559466
- DOI: http://doi.org/10.17998/jlc.2024.04.05
Abstract
- Backgrounds/Aims
To systematically evaluate inter-reader agreement in the assessment of individual liver imaging reporting and data system (LI-RADS) category M (LR-M) imaging features in computed tomography/magnetic resonance imaging (CT/MRI) LIRADS v2018, and to explore the causes of poor agreement in LR-M assignment.
Methods
Original studies reporting inter-reader agreement for LR-M features on multiphasic CT or MRI were identified using the MEDLINE, EMBASE, and Cochrane databases. The pooled kappa coefficient (κ) was calculated using the DerSimonian-Laird random-effects model. Heterogeneity was assessed using Cochran’s Q test and I2 statistics. Subgroup meta-regression analyses were conducted to explore the study heterogeneity.
Results
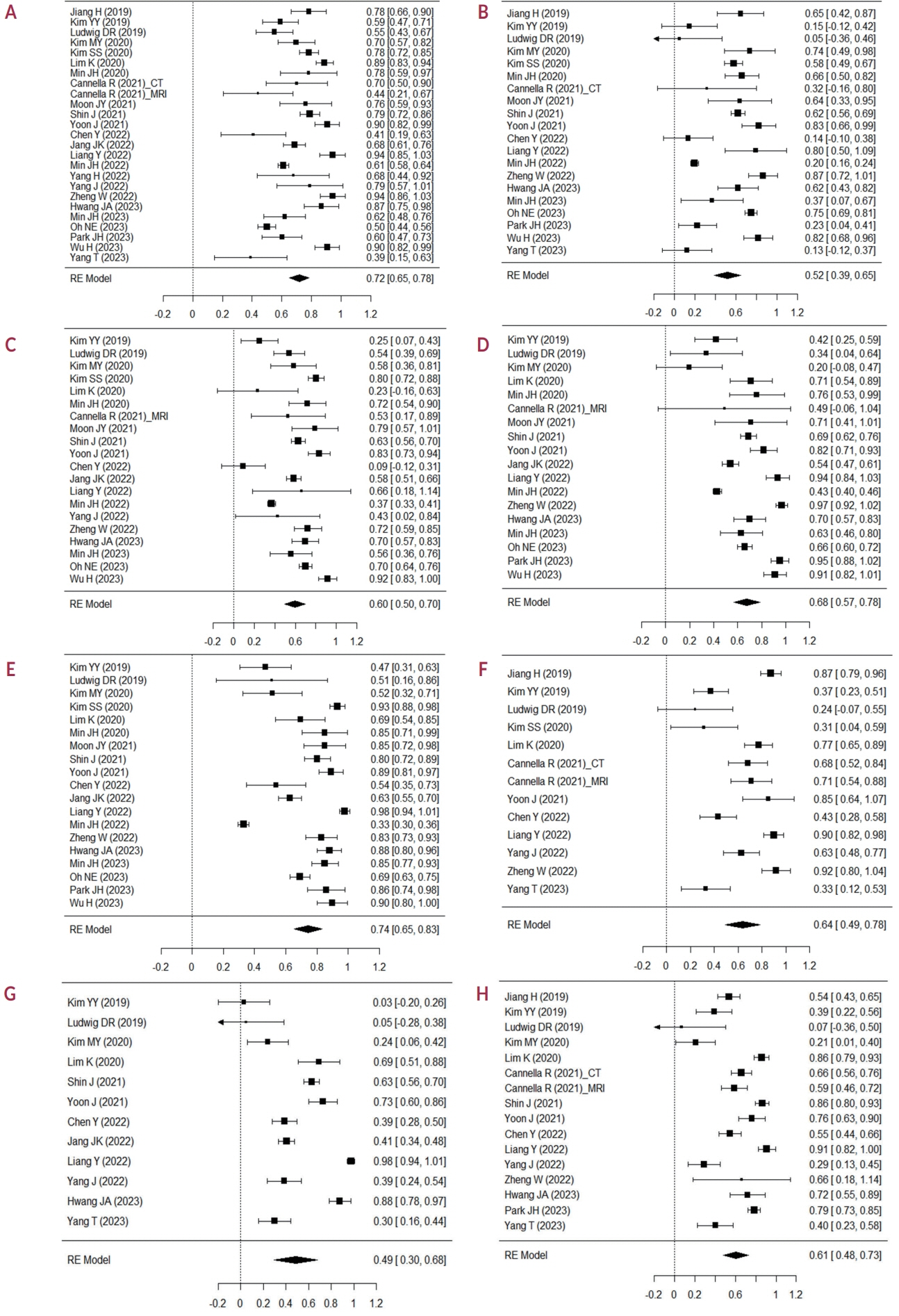
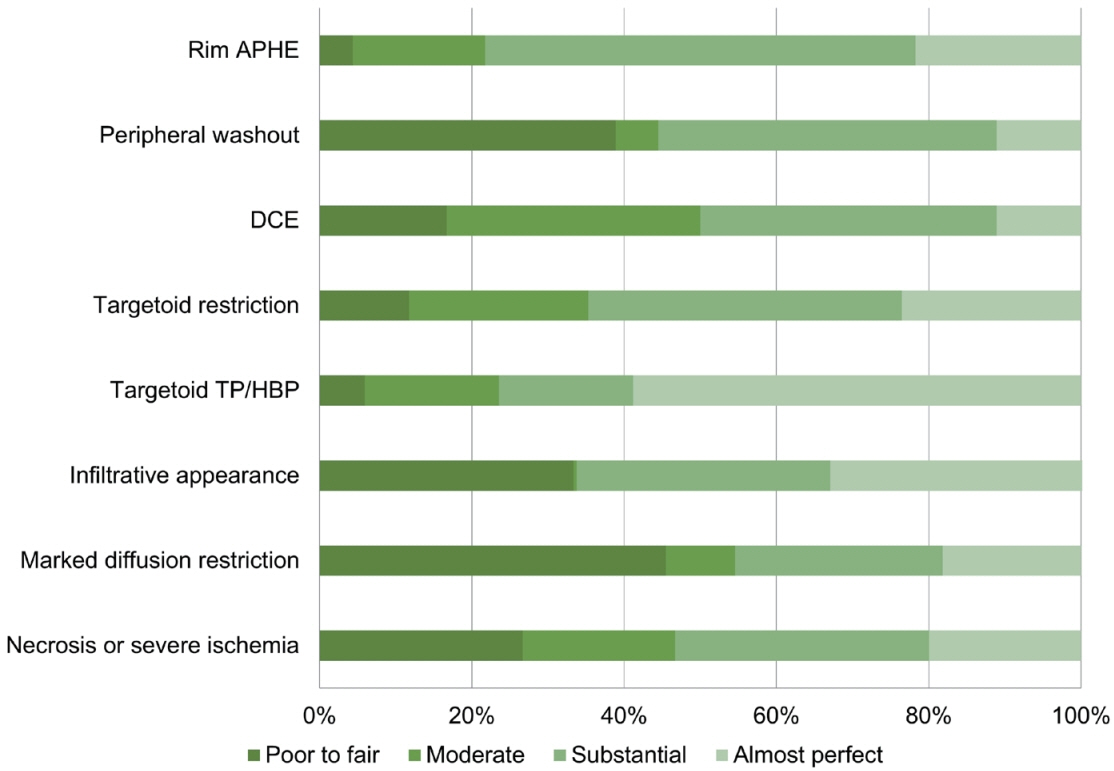
In total, 24 eligible studies with 5,163 hepatic observations were included. The pooled κ values were 0.72 (95% confidence interval [CI], 0.65-0.78) for rim arterial phase hyperenhancement, 0.52 (95% CI, 0.39-0.65) for peripheral washout, 0.60 (95% CI, 0.50-0.70) for delayed central enhancement, 0.68 (95% CI, 0.57-0.78) for targetoid restriction, 0.74 (95% CI, 0.65-0.83) for targetoid transitional phase/hepatobiliary phase appearance, 0.64 (95% CI, 0.49-0.78) for infiltrative appearance, 0.49 (95% CI, 0.30-0.68) for marked diffusion restriction, and 0.61 (95% CI, 0.48-0.73) for necrosis or severe ischemia. Substantial study heterogeneity was observed for all LR-M features (Cochran’s Q test, P<0.01; I2≥89.2%). Studies with a mean observation size of <3 cm, those performed using 1.5-T MRI, and those with multiple image readers, were significantly associated with poor agreement of LR-M features.
Conclusions
The agreement for peripheral washout and marked diffusion restriction was limited. The LI-RADS should focus on improving the agreement of LR-M features.
Keyword
Figure
Cited by 1 articles
-
Inter-reader agreement for LR-M imaging features: a premise for better imaging-based diagnosis in liver imaging
Jaeseung Shin
J Liver Cancer. 2024;24(2):124-125. doi: 10.17998/jlc.2024.08.06.
Reference
-
References
1. Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023; 78:1922–1965.
Article2. Kim DH, Choi SH, Park SH, Kim KW, Byun JH, Kim SY, et al. Liver imaging reporting and data system category M: a systematic review and meta-analysis. Liver Int. 2020; 40:1477–1487.3. Hwang SH, Rhee H. Radiologic features of hepatocellular carcinoma related to prognosis. J Liver Cancer. 2023; 23:143–156.
Article4. Hong CW, Chernyak V, Choi JY, Lee S, Potu C, Delgado T, et al. A multicenter assessment of inter-reader reliability of LI-RADS version 2018 for MRI and CT. Radiology. 2023; 307:e222855.5. Kim YY, Kim MJ, Kim EH, Roh YH, An C. Hepatocellular carcinoma versus other hepatic malignancy in cirrhosis: performance of LI-RADS version 2018. Radiology. 2019; 291:72–80.
Article6. Ludwig DR, Fraum TJ, Cannella R, Ballard DH, Tsai R, Naeem M, et al. Hepatocellular carcinoma (HCC) versus non-HCC: accuracy and reliability of Liver Imaging Reporting and Data System v2018. Abdom Radiol (NY). 2019; 44:2116–2132.
Article7. Kim MY, Joo I, Kang HJ, Bae JS, Jeon SK, Lee JM. LI-RADS M (LR-M) criteria and reporting algorithm of v2018: diagnostic values in the assessment of primary liver cancers on gadoxetic acid-enhanced MRI. Abdom Radiol (NY). 2020; 45:2440–2448.8. Kim SS, Lee S, Choi JY, Lim JS, Park MS, Kim MJ. Diagnostic performance of the LR-M criteria and spectrum of LI-RADS imaging features among primary hepatic carcinomas. Abdom Radiol (NY). 2020; 45:3743–3754.
Article9. Lim K, Kwon H, Cho J. Inter-reader agreement and imaging-pathology correlation of the LI-RADS M on gadoxetic acid-enhanced magnetic resonance imaging: efforts to improve diagnostic performance. Abdom Radiol (NY). 2020; 45:2430–2439.
Article10. Jang JK, Choi SH, Byun JH, Park SY, Lee SJ, Kim SY, et al. New strategy for Liver Imaging Reporting and Data System category M to improve diagnostic performance of MRI for hepatocellular carcinoma≤ 3.0 cm. Abdom Radiol (NY). 2022; 47:2289–2298.
Article11. Min JH, Lee MW, Park HS, Lee DH, Park HJ, Lee JE, et al. LI-RADS version 2018 targetoid appearances on gadoxetic acid-enhanced MRI: interobserver agreement and diagnostic performance for the differentiation of HCC and non-HCC malignancy. AJR Am J Roentgenol. 2022; 219:421–432.
Article12. Zheng W, Huang H, She D, Xiong M, Chen X, Lin X, et al. Added-value of ancillary imaging features for differentiating hepatocellular carcinoma from intrahepatic mass-forming cholangiocarcinoma on Gd-BOPTA-enhanced MRI in LI-RADS M. Abdom Radiol (NY). 2022; 47:957–968.13. Yang T, Wei H, Wu Y, Qin Y, Chen J, Jiang H, et al. Predicting histologic differentiation of solitary hepatocellular carcinoma up to 5 cm on gadoxetate disodium-enhanced MRI. Insights Imaging. 2023; 14:3.14. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000; 283:2008–2012.
Article15. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM; The PRISMA-DTA Group, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018; 319:388–396.
Article16. Kottner J, Audigé L, Brorson S, Donner A, Gajewski BJ, Hróbjartsson A, et al. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. J Clin Epidemiol. 2011; 64:96–106.
Article17. IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014; 14:25.18. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33:159–174.
Article19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.
Article20. Jiang H, Liu X, Chen J, Wei Y, Lee JM, Cao L, et al. Man or machine? Prospective comparison of the version 2018 EASL, LI-RADS criteria and a radiomics model to diagnose hepatocellular carcinoma. Cancer Imaging. 2019; 19:84.
Article21. Min JH, Kim SH, Hwang JA, Hyun SH, Ha SY, Choi SY, et al. Prognostic value of LI-RADS category on gadoxetic acid-enhanced MRI and 18FFDG PET-CT in patients with primary liver carcinomas. Eur Radiol. 2021; 31:3649–3660.
Article22. Cannella R, Burgio MD, Beaufrère A, Trapani L, Paradis V, Hobeika C, et al. Imaging features of histological subtypes of hepatocellular carcinoma: Implication for LI-RADS. JHEP Rep. 2021; 3:100380.23. Moon JY, Min JH, Kim YK, Cha D, Hwang JA, Ko SE, et al. Prognosis after curative resection of single hepatocellular carcinoma with a focus on LI-RADS targetoid appearance on preoperative gadoxetic acid-enhanced MRI. Korean J Radiol. 2021; 22:1786–1796.
Article24. Shin J, Lee S, Kim SS, Chung YE, Choi JY, Park MS, et al. Characteristics and early recurrence of hepatocellular carcinomas categorized as LR-M: comparison with those categorized as LR-4 or 5. J Magn Reson Imaging. 2021; 54:1446–1454.
Article25. Yoon J, Hwang JA, Lee S, Lee JE, Ha SY, Park YN. Clinicopathologic and MRI features of combined hepatocellular-cholangiocarcinoma in patients with or without cirrhosis. Liver Int. 2021; 41:1641–1651.
Article26. Chen Y, Qin Y, Wu Y, Wei H, Wei Y, Zhang Z, et al. Preoperative prediction of glypican-3 positive expression in solitary hepatocellular carcinoma on gadoxetate-disodium enhanced magnetic resonance imaging. Front Immunol. 2022; 13:973153.
Article27. Liang Y, Xu F, Wang Z, Tan C, Zhang N, Wei X, et al. A gadoxetic acidenhanced MRI-based multivariable model using LI-RADS v2018 and other imaging features for preoperative prediction of macrotrabecularmassive hepatocellular carcinoma. Eur J Radiol. 2022; 153:110356.28. Yang H, Han P, Huang M, Yue X, Wu L, Li X, et al. The role of gadoxetic acid-enhanced MRI features for predicting microvascular invasion in patients with hepatocellular carcinoma. Abdom Radiol (NY). 2022; 47:948–956.
Article29. Yang J, Jiang H, Xie K, Bashir MR, Wan H, Huang J, et al. Profiling hepatocellular carcinoma aggressiveness with contrast-enhanced ultrasound and gadoxetate disodium-enhanced MRI: an intra-individual comparative study based on the Liver Imaging Reporting and Data System. Eur J Radiol. 2022; 154:110397.
Article30. Hwang JA, Lee S, Lee JE, Yoon J, Choi SY, Shin J. LI-RADS category on MRI is associated with recurrence of intrahepatic cholangiocarcinoma after surgery: a multicenter study. J Magn Reson Imaging. 2023; 57:930–938.
Article31. Min JH, Lee MW, Rhim H, Han S, Song KD, Kang TW, et al. LI-RADS category is associated with treatment outcomes of small single HCC: surgical resection vs. radiofrequency ablation. Eur Radiol. 2024; 34:525–537.
Article32. Oh NE, Choi SH, Kim S, Lee H, Jang HJ, Byun JH, et al. Suboptimal performance of LI-RADS v2018 on gadoxetic acid-enhanced MRI for detecting hepatocellular carcinoma in liver transplant candidates. Eur Radiol. 2024; 34:465–474.33. Park JH, Park YN, Kim MJ, Park MS, Choi JY, Chung YE, et al. Steatotic hepatocellular carcinoma: association of MRI findings to underlying liver disease and clinicopathological characteristics. Liver Int. 2023; 43:1332–1344.
Article34. Wu H, Liang Y, Wang Z, Tan C, Yang R, Wei X, et al. Optimizing CT and MRI criteria for differentiating intrahepatic mass-forming cholangiocarcinoma and hepatocellular carcinoma. Acta Radiol. 2023; 64:926–935.
Article35. Kang JH, Choi SH, Lee JS, Park SH, Kim KW, Kim SY, et al. Interreader agreement of Liver Imaging Reporting and Data System on MRI: a systematic review and meta-analysis. J Magn Reson Imaging. 2020; 52:795–804.
Article36. van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, et al. Accuracy of the Liver Imaging Reporting and Data System in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy-a systematic review. Gastroenterology. 2019; 156:976–986.
Article37. Chernyak V, Sirlin CB. Editorial for “interreader agreement of Liver Imaging Reporting and Data System on MRI: a systematic review and meta analysis”. J Magn Reson Imaging. 2020; 52:805–806.38. American College of Radiology. LI-RADS lexicon [Internet]. Reston, VA (US): American College of Radiology;[cited 2024 Feb 4]. Available from: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LIRAD-SLexicon-Table.pdf.39. Fowler KJ, Bashir MR, Fetzer DT, Kitao A, Lee JM, Jiang H, et al. Universal liver imaging lexicon: imaging Atlas for research and clinical practice. Radiographics. 2023; 43:e220066.
Article40. Boll DT, Merkle EM. Imaging at higher magnetic fields: 3 T versus 1.5 T. Magn Reson Imaging Clin N Am. 2010; 18:549–564, xi-xii.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CT/MRI Liver Imaging Reporting and Data System (LI-RADS): Standardization, Evidence, and Future Direction
- Impact of the Liver Imaging Reporting and Data System on Research Studies of Diagnosing Hepatocellular Carcinoma Using MRI
- Combination of CT/MRI LI-RADS With Second-Line Contrast-Enhanced Ultrasound Using Sulfur Hexafluoride or Perfluorobutane for Diagnosing Hepatocellular Carcinoma in High-Risk Patients
- Diagnostic Image Feature and Performance of CT and Gadoxetic Acid Disodium-Enhanced MRI in Distinction of Combined HepatocellularCholangiocarcinoma from Hepatocellular Carcinoma
- LI-RADS Version 2018 Treatment Response Algorithm: Diagnostic Performance after Transarterial Radioembolization for Hepatocellular Carcinoma