J Liver Cancer.
2024 Sep;24(2):178-191. 10.17998/jlc.2024.03.25.
Outcomes of liver resection and transarterial chemoembolization in patients with multinodular BCLC-A hepatocellular carcinoma
- Affiliations
-
- 1Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Liver Transplantation and Hepatobiliary Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2559465
- DOI: http://doi.org/10.17998/jlc.2024.03.25
Abstract
- Backgrounds/Aims
This study aimed to compare the outcomes of liver resection (LR) and transarterial chemoembolization (TACE) in patients with multinodular hepatocellular carcinoma (HCC) within the Milan criteria who were not eligible for liver transplantation.
Methods
We retrospectively analyzed 483 patients with multinodular HCC within the Milan criteria, who underwent either LR or TACE as an initial therapy between 2013 and 2022. The overall survival (OS) in the entire population and recurrence-free survival (RFS) in patients who underwent LR and TACE and achieved a complete response were analyzed. Propensity score (PS) matching analysis was also used for a fair comparison of outcomes between the two groups.
Results
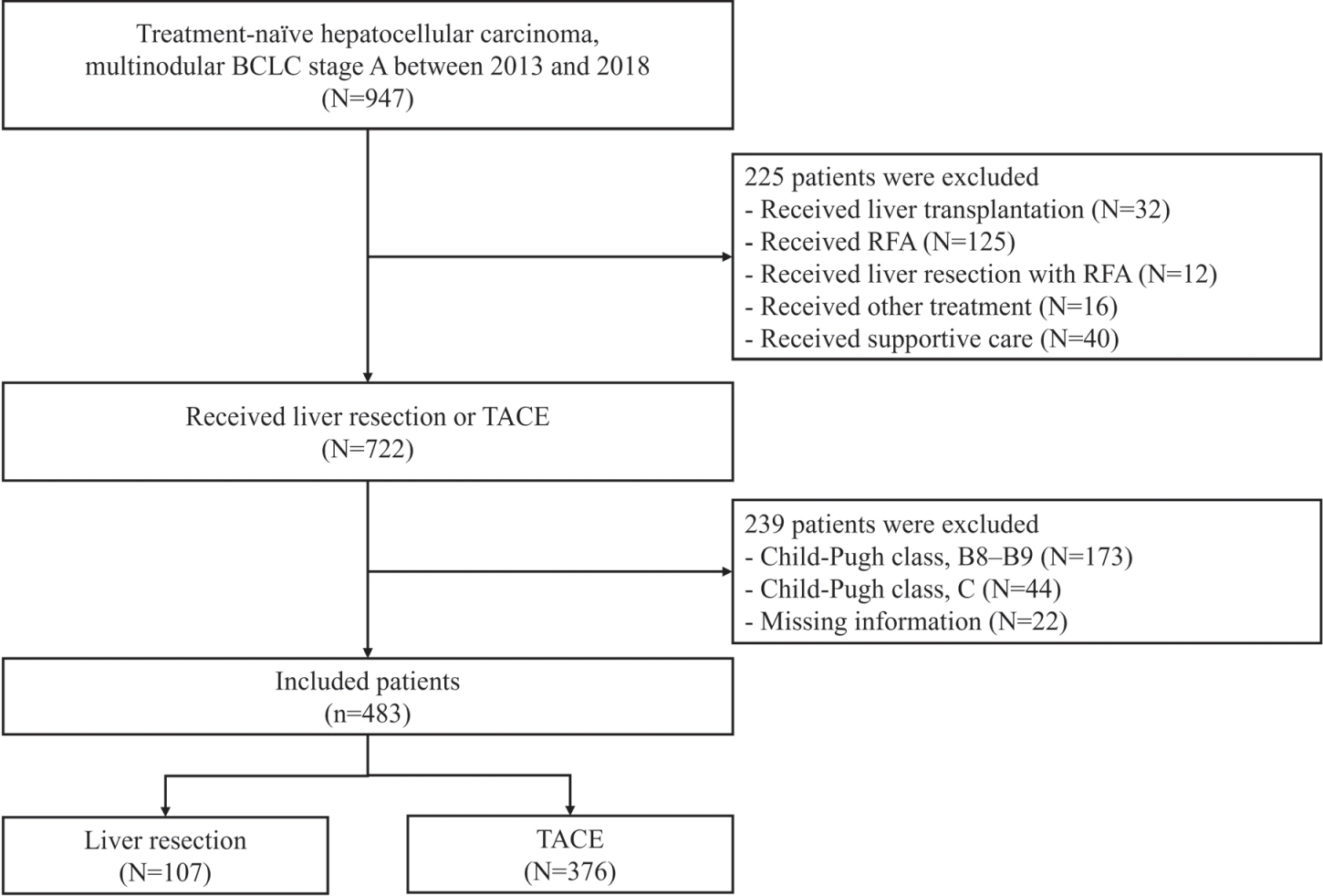
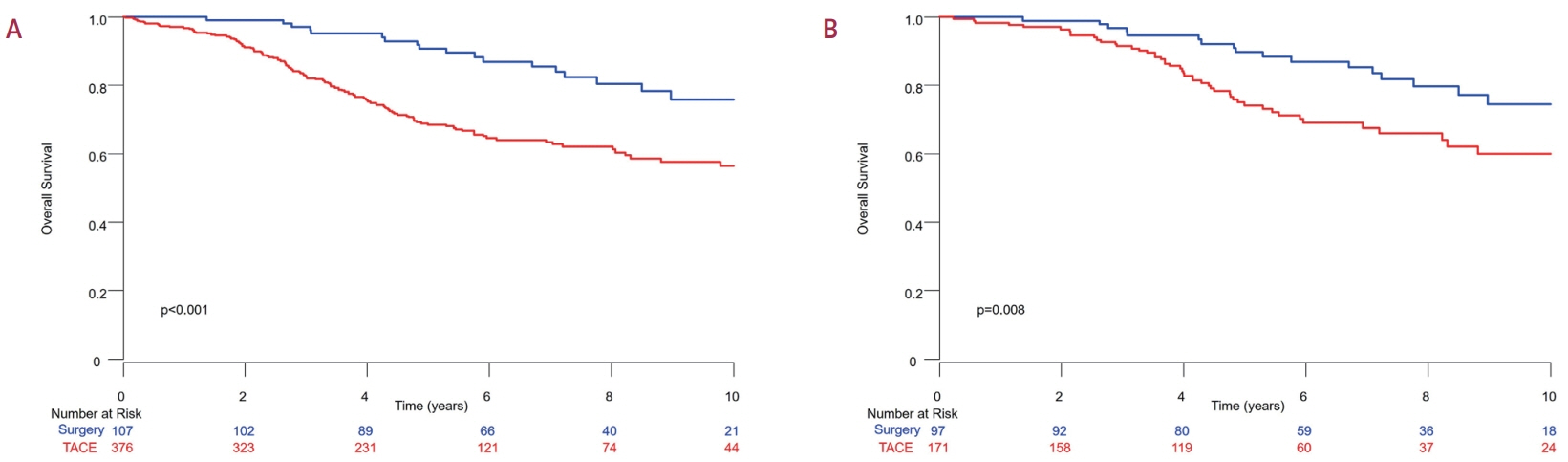
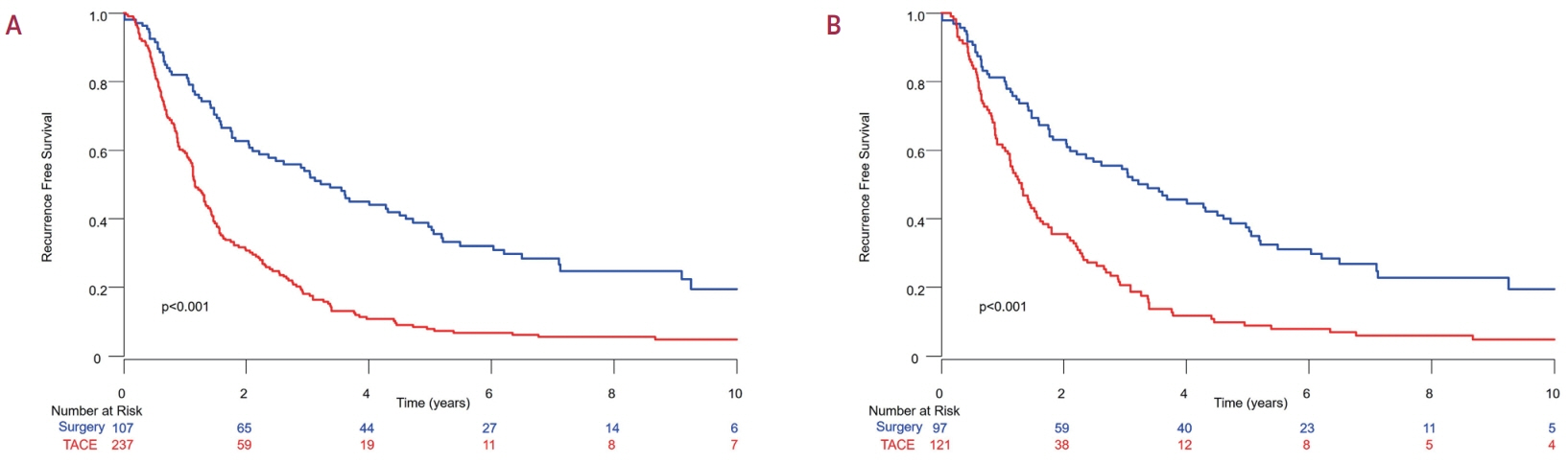
Among the 483 patients, 107 (22.2%) and 376 (77.8%) underwent LR and TACE, respectively. The median size of the largest tumor was 2.0 cm, and 72.3% of the patients had two HCC lesions. The median OS and RFS were significantly longer in the LR group than in the TACE group (P<0.01 for both). In the multivariate analysis, TACE (adjusted hazard ratio [aHR], 1.81 and aHR, 2.41) and large tumor size (aHR, 1.43 and aHR, 1.44) were significantly associated with worse OS and RFS, respectively. The PS-matched analysis also demonstrated that the LR group had significantly longer OS and RFS than the TACE group (PS<0.05).
Conclusions
In this study, LR showed better OS and RFS than TACE in patients with multinodular Barcelona Clinic Liver Cancer stage A HCC. Therefore, LR can be considered an effective treatment option for these patients.
Keyword
Figure
Cited by 1 articles
-
Exploring the role of liver resection as a first-line treatment option for multinodular BCLC-A hepatocellular carcinoma
Joo Hyun Oh, Dong Hyun Sinn
J Liver Cancer. 2024;24(2):126-128. doi: 10.17998/jlc.2024.08.08.
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249.
Article2. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022; 76:681–693.
Article3. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.4. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022; 28:583–705.5. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003; 38:200–207.
Article6. Poon RTP, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000; 232:10–24.
Article7. Hidaka M, Hara T, Soyama A, Adachi T, Matsushima H, Tanaka T, et al. Long-term outcomes of living-donor liver transplantation, hepatic resection, and local therapy for hepatocellular carcinoma with three <3- cm nodules in a single institute. JGH Open. 2022; 6:539–546.
Article8. Fukami Y, Kaneoka Y, Maeda A, Kumada T, Tanaka J, Akita T, et al. Liver resection for multiple hepatocellular carcinomas: a Japanese nationwide survey. Ann Surg. 2020; 272:145–154.9. Terminology Committee of the International Hepato-Pancreato-Biliary Association. The Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000; 2:333–339.10. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011; 149:713–724.11. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: fiveyear experience. Ann Surg. 2009; 250:187–196.12. Choi J, Lee D, Shim JH, Kim KM, Lim YS, Lee YS, et al. Evaluation of transarterial chemoembolization refractoriness in patients with hepatocellular carcinoma. PLoS One. 2020; 15:e0229696.
Article13. Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011; 55:1309–1316.
Article14. Lu L, Zheng P, Wu Z, Chen X. Hepatic resection versus transarterial chemoembolization for intermediate-stage hepatocellular carcinoma: a cohort study. Front Oncol. 2021; 11:618937.
Article15. Yue YY, Zhou WL. Hepatic resection is associated with improved long-term survival compared to radio-frequency ablation in patients with multifocal hepatocellular carcinoma. Front Oncol. 2020; 10:110.16. Nakagawa S, Beppu T, Okabe H, Sakamoto K, Kuroki H, Mima K, et al. Triple positive tumor markers predict recurrence and survival in early stage hepatocellular carcinoma. Hepatol Res. 2014; 44:964–974.
Article17. Suh SW, Lee KW, Lee JM, You T, Choi Y, Kim H, et al. Prediction of aggressiveness in early-stage hepatocellular carcinoma for selection of surgical resection. J Hepatol. 2014; 60:1219–1224.
Article18. Kwon JH, Lee JW, Lee JW, Lee YJ. Effects of anatomical or non-anatomical resection of hepatocellular carcinoma on survival outcome. J Clin Med. 2022; 11:1369.
Article19. Rhim H, Lim HK. Radiofrequency ablation of hepatocellular carcinoma: pros and cons. Gut Liver. 2010; 4 Suppl 1:S113–S118.
Article20. Bargellini I, Bozzi E, Campani D, Carrai P, De Simone P, Pollina L, et al. Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur J Radiol. 2013; 82:e212–e218.21. Burgio MD, Ronot M, Bruno O, Francoz C, Paradis V, Castera L, et al. Correlation of tumor response on computed tomography with pathological necrosis in hepatocellular carcinoma treated by chemoembolization before liver transplantation. Liver Transpl. 2016; 22:1491–1500.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Outcome after Transarterial Chemoembolization; Refinement of Barcelona Clinic Liver Cancer Stage-B from Eastern Point of View
- Is Transarterial Chemoembolization Only Treatment Option in Patients with Intermediate Stage of Hepatocellular Carcinoma?: in Perspectives of Surgery
- Recent technical advances in conventional transarterial chemoembolization for hepatocellular carcinoma in Japan
- An A to Z of Lipiodol Beyond the Clinical Practice in the Management of Hepatocellular Carcinoma
- Transarterial Chemoembolization in Patients with Hepatocellular Carcinoma and Extrahepatic Metastasis