Ann Pediatr Endocrinol Metab.
2024 Aug;29(4):250-257. 10.6065/apem.2346178.089.
Impacts of circulating cytokine levels and gene polymorphism predisposition on type 1 diabetes mellitus
- Affiliations
-
- 1Faculty of Medicine, Al-Baha University, Al-Baha, Saudi Arabia
- 2Faculty of Science, Al-Baha University, Al-Baha, Saudi Arabia
- 3Genetic Institute, Sadat City University, Egypt
- 4Demietta Faculty of Medicine, Al-Azhar University, Cairo, Egypt
- KMID: 2559453
- DOI: http://doi.org/10.6065/apem.2346178.089
Abstract
- Purpose
A wide range of cytokines has been demonstrated to be involved in the etiology of type 1 diabetes mellitus (T1DM). Gene polymorphisms may potentially contribute to a hereditary predisposition toward circulating cytokine levels as (high, intermediate, or low) since they can affect cytokine production or function. The aim of this study was to investigate the roles of cytokine levels and the association of single-nucleotide polymorphisms (SNPs) within cytokine genes with T1DM in Saudi children.
Methods
Totals of 91 well-characterized T1DM patients and 91 T1DM-free control subjects were enrolled in this study.
Results
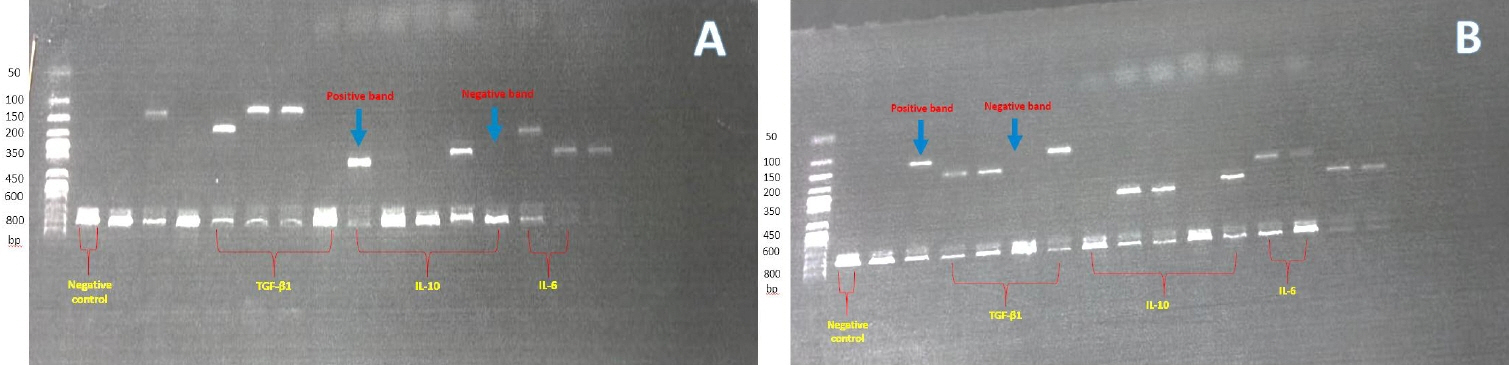
The levels of 3 circulating cytokines (transforming growth factor [TGF]-β1, interleukin [IL]-10, and IL-6) and 6 SNPs in 3 cytokine genes (TGF-β1 [rs1800470 and rs1800471], IL-10 [rs1800896, rs1800871, and rs1800872], and IL-6 [rs1800795]) that contribute to genetic susceptibility were measured by enzyme-linked immunosorbent assay and polymerase chain reaction with sequence-specific primers. Our fn dings show that TGF-β1 serum levels were signifcantly lower in the children with T1DM than in the control participants. The TGF-β1 genotypes with a high-production phenotype were signifcantly less frequent and those with a lowproduction phenotype were signifcantly more frequent in the children with T1DM compared to the control participants. respectively. Furthermore, the IL-6 genotype frequency with low level of IL-6 production were signifcantly increased in the T1DM group compared to the control group. Moreover, our data demonstrated no appreciable diferences in circulating serum level or genotype and phenotype of IL- 10 between the patients and controls.
Conclusion
This kind of measurement, which considers the prediction of T1DM, may be useful in assessing the severity of T1DM and susceptibility to T1DM among Saudi children.
Keyword
Figure
Reference
-
References
1. Rastogi I, Jeon D, Moseman JE, Muralidhar A, Potluri HK, McNeel DG. Role of B cells as antigen presenting cells. Front Immunol. 2022; 13:954936.2. Magnuson AM, Thurber GM, Kohler RH, Weissleder R, Mathis D, Benoist C. Population dynamics of isletinfiltrating cells in autoimmune diabetes. Proc Natl Acad Sci U S A. 2015; 112:1511–6.
Article3. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018; 391:2449–62.
Article4. Nepom GT, Ehlers M, Mandrup-Poulsen T. Anti-cytokine therapies in T1D: Concepts and strategies. Clin Immunol. 2013; 149:279–85.
Article5. Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010; 207:1871–8.
Article6. Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009; 32:1244–9.
Article7. Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013; 1:295–305.
Article8. Dong S, Hiam-Galvez KJ, Mower y CT, Herold KC, Gitelman SE, Esensten JH, et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight. 2021; 6:e147474.
Article9. You S, Thieblemont N, Alyanakian MA, Bach JF, Chatenoud L. Transforming growth factor-beta and T-cell-mediated immunoregulation in the control of autoimmune diabetes. Immunol Rev. 2006; 212:185–202.
Article10. Ninic A, Bojanin D, Sopic M, Mihajlovic M, Munjas J, Milenkovic T, et al. Transforming growth factor-beta1 and receptor for advanced glycation end products gene expression and protein levels in adolescents with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol. 2021; 13:61–71.11. Dhaouadi T, Sfar I, Bardi R, Jendoubi-Ayed S, Abdallah TB, Ayed K, et al. Cytokine gene polymorphisms in kidney transplantation. Transplant Proc. 2013; 45:2152–7.
Article12. Wang W, Sung N, Gilman-Sachs A, Kwak-Kim J. T Helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front Immunol. 2020; 11:2025.
Article13. Dasgupta S, Dasgupta S, Bandyopadhyay M. Regulatory B cells in infection, inflammation, and autoimmunity. Cell Immunol. 2020; 352:104076.
Article14. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015; 42:607–12.
Article15. Daien CI, Gailhac S, Mura T, Audo R, Combe B, Hahne M, et al. Regulatory B10 cells are decreased in patients with rheumatoid arthritis and are inversely correlated with disease activity. Arthritis Rheumatol. 2014; 66:2037–46.
Article16. Yin YW, Sun QQ, Zhang BB, Hu AM, Wang Q, Liu HL, et al. The lack of association between interleukin-6 gene -174 G/C polymorphism and the risk of type 1 diabetes mellitus: a meta-analysis of 18,152 subjects. Gene. 2013; 515:461–5.17. Ali YBM, El-Gahel HE, Abdel-Hakem NE, Gadalla ME, ElHefnawy MH, El-Shahat M. Association between IL-18 and IL-6 gene polymorphisms and the risk of T1D in Egyptian children. J Diabetes Metab Disord. 2021; 20:439–46.
Article18. American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016; 39 Suppl 1:S13–22. Erratum in: Diabetes Care 2016;39:1653.19. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988; 16:1215.
Article20. Alper CM, Winther B, Hendley JO, Doyle WJ. Cytokine polymorphisms predict the frequency of otitis media as a complication of rhinovirus and RSV infections in children. Eur Arch Otorhinolaryngol. 2009; 266:199–205.
Article21. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019; 157:107843.
Article22. Ichinose K, Kawasaki E, Eguchi K. Recent advancement of understanding pathogenesis of type 1 diabetes and potential relevance to diabetic nephropathy. Am J Nephrol. 2007; 27:554–64.23. Jakus V, Sapak M, Kostolanska J. Circulating TGF-beta1, glycation, and oxidation in children with diabetes mellitus type 1. Exp Diabetes Res. 2012; 2012:510902.24. Ide A, Kawasaki E, Abiru N, Sun F, Fukushima T, Ishii R, et al. Interleukin-10 gene promoter region polymorphisms in patients with type 1 diabetes and autoimmune thyroid disease. Ann N Y Acad Sci. 2003; 1005:344–7.
Article25. Urcelay E, Santiago JL, de la Calle H, Martinez A, Figueredo A, Fernandez-Arquero M, et al. Interleukin-10 polymorphisms in Spanish type 1 diabetes patients. Genes Immun. 2004; 5:306–9.
Article26. Siek iera U, Jarosz-Chob ot P, Janusz J, Ko eh ler B. Polimorfizm genów cytokinowych TNF-alpha (308 A/G), IL-10 (1082 A/T 819 C/T 592 A/C), IL-6 (174 C/G), IFN-gamma (874 A/T); określenie genetycznie uwarunkowanego poziomu syntezy cytokin w grupie dzieci chorych z cukrzyca typu 1 [Polymorphism of TNF-alpha (308 A/G), IL-10 (1082 A/G, 819 C/T 592 A/C), IL-6 (174 G/C), and IFN-gamma (874 A/T); genetically conditioned cytokine synthesis level in children with diabetes type 1]. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2002; 8:29–34. Polish.27. Gouda W, Mageed L, Abd El Dayem SM, Ashour E, Afify M. Evaluation of pro-inflammatory and anti-inflammatory cytokines in type 1 diabetes mellitus. Bull Natl Res Cent. 2018; 42:14.
Article28. He JS, Xie PS, Luo DS, Sun CJ, Zhang YG, Liu FX. Role of immune dysfunction in pathogenesis of type 1 diabetes mellitus in children. Asian Pac J Trop Med. 2014; 7:823–6.
Article29. Chen YL, Qiao YC, Pan YH, Xu Y, Huang YC, Wang YH, et al. Correlation between serum interleukin-6 level and type 1 diabetes mellitus: a systematic review and meta-analysis. Cytokine. 2017; 94:14–20.
Article30. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016; 893:1–19.
Article31. Starkie RL, Arkinstall MJ, Koukoulas I, Hawley JA, Febbraio MA. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J Physiol. 2001; 533:585–91.
Article32. Sandler S, Bendtzen K, Eizirik DL, Welsh M. Interleukin-6 affects insulin secretion and glucose metabolism of rat pancreatic islets in vitro. Endocrinology. 1990; 126:1288–94.33. Suzuki M, Saito M, Nagai T, Saeki H, Kazatani Y. Systemic versus coronary levels of inflammation in acute coronary syndromes. Angiology. 2006; 57:459–63.
Article34. Jahromi MM, Millward BA, Demaine AG. A polymorphism in the promoter region of the gene for interleukin-6 is associated with susceptibility to type 1 diabetes mellitus. J Interferon Cytokine Res. 2000; 20:885–8.
Article35. Choi SE, Choi KM, Yoon IH, Shin JY, Kim JS, Park WY, et al. IL-6 protects pancreatic islet beta cells from proinflammatory cytokines-induced cell death and functional impairment in vitro and in vivo. Transpl Immunol. 2004; 13:43–53.
Article36. Cooper JD, Smyth DJ, Bailey R, Payne F, Downes K, Godfrey LM, et al. The candidate genes TAF5L, TCF7, PDCD1, IL6 and ICAM1 cannot be excluded from having effects in type 1 diabetes. BMC Med Genet. 2007; 8:71.37. Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008; 14:109–19.
Article38. Cartwright NH, Keen LJ, Demaine AG, Hurlock NJ, McGonigle RJ, Rowe PA, et al. A study of cytokine gene polymorphisms and protein secretion in renal transplantation. Transpl Immunol. 2001; 8:237–44.
Article39. Reuss E, Fimmers R, Kruger A, Becker C, Rittner C, Hohler T. Differential regulation of interleukin-10 production by genetic and environmental factors--a twin study. Genes Immun. 2002; 3:407–13.
Article40. Tayel MY, Nazir A, Abdelhamid IM, Helmy MAS, Zaki NE, Elsharkawy NS, et al. TNF-α -308 G>A and IL10 -1082A>G polymorphisms as potential risk factors for lymphoproliferative disorders in autoimmune rheumatic diseases. Egypt J Med Hum Genet. 2020; 21:2.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autoantibodies and Polymorphism of the TNF gene in Autoimmune Diabetes Mellitus
- The Role of Plasma Adiponectin and Polymorphism of Adiponectin Gene in the Development of Type 2 Diabetes Mellitus
- Non-Insulin Dependent Diabetes Mellitus andbeta3-Adrenergic Receptor Gene Polymorphism
- Usefulness of Fibrinogen B beta 448 Polymorphism as a Marker of Cerebral Infarction in Patients with Diabetes Mellitus
- Sulwon Lecture 2009: The Search for Genetic Risk Factors of Type 2 Diabetes Mellitus