Ann Pediatr Endocrinol Metab.
2024 Aug;29(4):211-219. 10.6065/apem.2346238.119.
The role of MicroRNAs as fine-tuners in the onset of puberty: a comprehensive review
- Affiliations
-
- 1Department of Pediatrics, Soonchunhyang University College of Medicine, Cheonan, Korea
- 2Department of Pediatrics, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- KMID: 2559448
- DOI: http://doi.org/10.6065/apem.2346238.119
Abstract
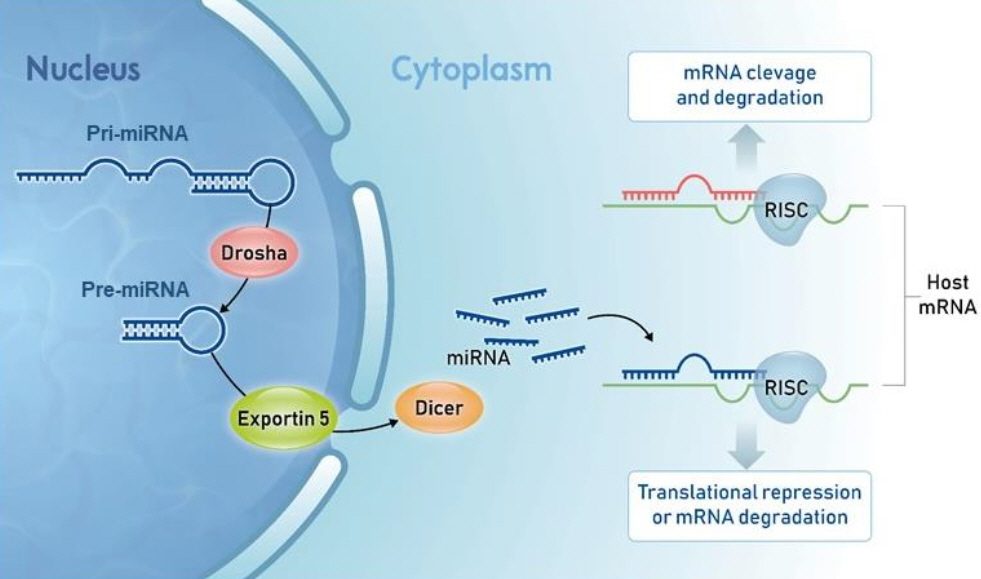
- MicroRNA (miRNA) are small, noncoding RNA molecules that play pivotal roles in gene expression, various biological processes, and development of disease. MiRNAs exhibit distinct expression patterns depending on time points and tissues, indicating their relevance to the development, differentiation, and somatic growth of organisms. MiRNAs are also involved in puberty onset and fertility. Although puberty is a universal stage in the life cycles of most organisms, the precise mechanisms initiating this process remain elusive. Genetic, hormonal, nutritional, environmental, and epigenetic factors are presumed contributors. The intricate regulation of puberty during growth also suggests that miRNAs are involved. This study aims to provide insight into the understanding of miRNAs roles in the initiation of puberty by reviewing the existing research.
Keyword
Figure
Reference
-
References
1. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003; 24:668–93.
Article2. Mancini A, Magnotto JC, Abreu AP. Genetics of pubertal timing. Best Pract Res Clin Endocrinol Metab. 2022; 36:101618.
Article3. Palmert MR, Hirschhorn JN. Genetic approaches to stature, pubertal timing, and other complex traits. Mol Genet Metab. 2003; 80:1–10.
Article4. Juul A, Teilmann G, Scheike T, Hertel NT, Holm K, Laursen EM, et al. Pubertal development in Danish children: comparison of recent European and US data. Int J Androl. 2006; 29:247–55. discussion 286-90.
Article5. Livadas S, Chrousos GP. Molecular and environmental mechanisms regulating puberty initiation: an integrated approach. Front Endocrinol (Lausanne). 2019; 10:828.
Article6. Kim MR, Jung MK, Jee HM, Ha EK, Lee S, Han MY, et al. The association between phthalate exposure and pubertal development. Eur J Pediatr. 2024; 183:1675–82.
Article7. Peper JS, Burke SM, Wierenga LM. Sex differences and brain development during puberty and adolescence. Handb Clin Neurol. 2020; 175:25–54.8. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116:281–97.9. Rajman M, Schratt G. MicroRNAs in neural development: from master regulators to fine-tuners. Development. 2017; 144:2310–22.
Article10. Amar L, Benoit C, Beaumont G, Vacher CM, Crepin D, Taouis M, et al. MicroRNA expression profiling of hypothalamic arcuate and paraventricular nuclei from single rats using Illumina sequencing technology. J Neurosci Methods. 2012; 209:134–43.
Article11. Lettre G, Jackson AU, Gieger C, Schumacher FR, Berndt SI, Sanna S, et al. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet. 2008; 40:584–91.
Article12. He C, Kraft P, Chen C, Buring JE, Paré G, Hankinson SE, et al. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet. 2009; 41:724–8.
Article13. Ong KK, Elks CE, Li S, Zhao JH, Luan J, Andersen LB, et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet. 2009; 41:729–33.
Article14. Tommiska J, Wehkalampi K, Vaaralahti K, Laitinen EM, R aivio T, Dunkel L. LIN28B in constitutional delay of growth and puberty. J Clin Endocrinol Metab. 2010; 95:3063–6.
Article15. Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984; 226:409–16.
Article16. Yang DH, Moss EG. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr Patterns. 2003; 3:719–26.
Article17. Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008; 18:505–16.
Article18. Chang TC, Zeitels LR, Hwang HW, Chivukula RR, Wentzel EA, Dews M, et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci U S A. 2009; 106:3384–9.
Article19. Viswanathan SR, Daley GQ. Lin28: a microRNA regulator with a macro role. Cell. 2010; 140:445–9.
Article20. Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci U S A. 2009; 106:3207–12.21. Zhu H, Shah S, Shyh-Chang N, Shinoda G, Einhorn WS, Viswanathan SR, et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet. 2010; 42:626–30.
Article22. Sachdeva M, Mo YY. miR-145-mediated suppression of cell growth, invasion and metastasis. Am J Transl Res. 2010; 2:170–80.23. Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000; 403:901–6.
Article24. Sangiao-Alvarellos S, Manfredi-Lozano M, Ruiz-Pino F, Navarro VM, Sánchez-Garrido MA, Leon S, et al. Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty. Endocrinology. 2013; 154:942–55.
Article25. Gaytan F, Sangiao-Alvarellos S, Manfredi-Lozano M, García-Galiano D, Ruiz-Pino F, Romero-Ruiz A, et al. Distinct expression patterns predict differential roles of the miRNA-binding proteins, Lin28 and Lin28b, in the mouse testis: studies during postnatal development and in a model of hypogonadotropic hypogonadism. Endocrinology. 2013; 154:1321–36.
Article26. Chen T, Chen C, Wu H, Chen X, Xie R, Wang F, et al. Overexpression of p53 accelerates puberty in high-fat diet-fed mice through Lin28/let-7 system. Exp Biol Med (Maywood). 2021; 246:66–71.
Article27. Garaffo G, Conte D, Provero P, Tomaiuolo D, Luo Z, Pinciroli P, et al. The Dlx5 and Foxg1 transcription factors, linked via miRNA-9 and -200, are required for the development of the olfactory and GnRH system. Mol Cell Neurosci. 2015; 68:103–19.
Article28. Messina A, Langlet F, Chachlaki K, Roa J, Rasika S, Jouy N, et al. A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nat Neurosci. 2016; 19:835–44.
Article29. Nolan K, Mitchem MR, Jimenez-Mateos EM, Henshall DC, Concannon CG, Prehn JH. Increased expression of microRNA-29a in ALS mice: functional analysis of its inhibition. J Mol Neurosci. 2014; 53:231–41.
Article30. Zhang L, Cai Z, Wei S, Zhou H, Zhou H, Jiang X, et al. MicroRNA expression profiling of the porcine developing hyp ot hal amus and pituit ar y tissue. Int J Mol S ci. 2013; 14:20326–39.31. Takeda T, Tanabe H. Lifespan and reproduction in brain-specific miR-29-knockdown mouse. Biochem Biophys Res Commun. 2016; 471:454–8.
Article32. Ripa R, Dolfi L, Terrigno M, Pandolfini L, Savino A, Arcucci V, et al. MicroRNA miR-29 controls a compensatory response to limit neuronal iron accumulation during adult life and aging. BMC Biol. 2017; 15:9.
Article33. Keynes R, Lumsden A. Segmentation and the origin of regional diversity in the vertebrate central nervous system. Neuron. 1990; 4:1–9.
Article34. Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990; 5:1–10.
Article35. Li X, Xiao J, Fan Y, Yang K, Li K, Wang X, et al. miR-29 family regulates the puberty onset mediated by a novel Gnrh1 transcription factor TBX21. J Endocrinol. 2019; 242:185–97.
Article36. Zhang N, Lin JK, Chen J, Liu XF, Liu JL, Luo HS, et al. MicroRNA 375 mediates the signaling pathway of corticotropin-releasing factor (CRF) regulating pro-opiomelanocortin (POMC) expression by targeting mitogen-activated protein k inas e 8. J Biol Chem. 2013; 288:10361–73.37. Li H, Li X, Zhang D, Li J, Cui S. MiR-375 potentially enhances GnRH expression by targeting Sp1 in GT1-7 cells. In Vitro Cell Dev Biol Anim. 2021; 57:438–47.
Article38. Verduci L, Simili M, Rizzo M, Mercatanti A, Evangelista M, Mariani L, et al. MicroRNA (miRNA)-mediated interaction between leukemia/lymphoma-related factor (LRF) and alternative splicing factor/splicing factor 2 (ASF/SF2) affects mouse embryonic fibroblast senescence and apoptosis. J Biol Chem. 2010; 285:39551–63.
Article39. Karni R, Hippo Y, Lowe SW, Krainer AR. The splicing-factor oncoprotein SF2/ASF activates mTORC1. Proc Natl Acad Sci U S A. 2008; 105:15323–7.
Article40. Roa J, Garcia-Galiano D, Varela L, Sánchez-Garrido MA, Pineda R, Castellano JM, et al. The Mammalian Target of Rapamycin as Novel Central Regulator of Puberty Onset via Modulation of Hypothalamic Kiss1 System. Endocrinology. 2009; 150:5016–26.
Article41. Zhou Y, Tong L, Wang M, Chang X, Wang S, Li K, et al. miR-505-3p is a repressor of the puberty onset in female mice. J Endocrinol. 2018; Dec. 1. JOE-18-0533.R2. doi: 10.1530/JOE-18-0533. [Epub].42. Plant TM. The neurobiological mechanism underlying hypothalamic GnRH pulse generation: the role of kisspeptin neurons in the arcuate nucleus. F1000Res. 2019; 8:F1000 Faculty Rev-982.
Article43. Li X, Xiao J, Li K, Zhou Y. MiR-199-3p modulates the onset of puberty in rodents probably by regulating the expression of Kiss1 via the p38 MAPK pathway. Mol Cell Endocrinol. 2020; 518:110994.
Article44. Urbanski HF, Ojeda SR. A role for N-methyl-D-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology. 1990; 126:1774–6.
Article45. Mahesh VB, Brann DW. Regulatory role of excitatory amino acids in reproduction. Endocrine. 2005; 28:271–80.
Article46. Maffucci JA, Noel ML, Gillette R, Wu D, Gore AC. Age- and hormone-regulation of N-methyl-D-aspartate receptor subunit NR2b in the anteroventral periventricular nucleus of the female rat: implications for reproductive senescence. J Neuroendocrinol. 2009; 21:506–17.47. Bourguignon JP, Gérard A, Alvarez Gonzalez ML, Franchimont P. Neuroendocrine mechanism of onset of puberty. Sequential reduction in activity of inhibitory and facilitatory N-methyl-D-aspartate receptors. J Clin Invest. 1992; 90:1736–44.
Article48. Gore AC, Wu TJ, Rosenberg JJ, Roberts JL. Gonadotropin-releasing hormone and NMDA receptor gene expression and colocalization change during puberty in female rats. J Neurosci. 1996; 16:5281–9.
Article49. Ju M, Yang L, Zhu J, Chen Z, Zhang M, Yu J, et al. MiR-664- 2 impacts pubertal development in a precocious-puberty rat model through targeting the NMDA receptor-1†. Biol Reprod. 2019; 100:1536–48.50. Abreu AP, Dauber A, Macedo DB, Noel SD, Brito VN, Gill JC, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013; 368:2467–75.
Article51. Hagen CP, Sørensen K, Mieritz MG, Johannsen TH, Almstrup K, Juul A. Circulating MKRN3 levels decline prior to pubertal onset and through puberty: a longitudinal study of healthy girls. J Clin Endocrinol Metab. 2015; 100:1920–6.
Article52. Busch AS, Hagen CP, Almstrup K, Juul A. Circulating MKRN3 levels decline during puberty in healthy boys. J Clin Endocrinol Metab. 2016; 101:2588–93.
Article53. Jong MT, Carey AH, Caldwell KA, Lau MH, Handel MA, Driscoll DJ, et al. Imprinting of a RING zinc-finger encoding gene in the mouse chromosome region homologous to the Prader-Willi syndrome genetic region. Hum Mol Genet. 1999; 8:795–803.
Article54. Heras V, Sangiao-Alvarellos S, Manfredi-Lozano M, Sanchez-Tapia MJ, Ruiz-Pino F, Roa J, et al. Hypothalamic miR-30 regulates puberty onset via repression of the puberty-suppressing factor, Mkrn3. PLoS Biol. 2019; 17:e3000532.
Article55. Mørup N, Stakaitis R, Main AM, Golubickaite I, Hagen CP, Juul A, et al. Circulating levels and the bioactivity of miR-30b increase during pubertal progression in boys. Front Endocrinol (Lausanne). 2023; 14:1120115.
Article56. Wijchers PJ, Festenstein RJ. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends Genet. 2011; 27:132–40.
Article57. Morgan CP, Bale TL. Sex differences in microRNA regulation of gene expression: no smoke, just miRs. Biol Sex Differ. 2012; 3:22.
Article58. Hou H, Chan C, Yuki KE, Sokolowski D, Roy A, Qu R, et al. Postnatal developmental trajectory of sex-biased gene expression in the mouse pituitary gland. Biol Sex Differ. 2022; 13:57.
Article59. Hossain MM, Ghanem N, Hoelker M, Rings F, Phatsara C, Tholen E, et al. Identification and characterization of miRNAs expressed in the bovine ovary. BMC Genomics. 2009; 10:443.
Article60. Mishima T, Takizawa T, Luo SS, Ishibashi O, Kawahigashi Y, Mizuguchi Y, et al. MicroRNA (miRNA) cloning analysis reveals sex differences in miRNA expression profiles between adult mouse testis and ovary. Reproduction. 2008; 136:811–22.
Article61. Torley KJ, da Silveira JC, Smith P, Anthony RV, Veeramachaneni DN, Winger QA, et al. Expression of miRNAs in ovine fetal gonads: potential role in gonadal differentiation. Reprod Biol Endocrinol. 2011; 9:2.
Article62. Bannister SC, Tizard ML, Doran TJ, Sinclair AH, Smith CA. Sexually dimorphic microRNA expression during chicken embryonic gonadal development. Biol Reprod. 2009; 81:165–76.63. Yang Q, Hua J, Wang L, Xu B, Zhang H, Ye N, et al. MicroRNA and piRNA profiles in normal human testis detected by next generation sequencing. PLoS One. 2013; 8:e66809.
Article64. Ziats MN, Rennert OM. Identification of differentially expressed microRNAs across the developing human brain. Mol Psychiatry. 2014; 19:848–52.
Article65. Dai R, Ahmed SA. S exual dimorphism of miRNA expression: a new perspective in understanding the sex bias of autoimmune diseases. Ther Clin Risk Manag. 2014; 10:151–63.66. Han W, Zhu Y, Su Y, Li G, Qu L, Zhang H, et al. High-throughput sequencing reveals circulating miRNAs as potential biomarkers for measuring puberty onset in chicken (Gallus gallus). PLoS One. 2016; 11:e0154958.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetic and epigenetic aspects of the KISS1 and KISS1R genes in pubertal development and central precocious puberty: A review
- Abnormal pubertal development in Korean adolescent girls
- Effect of melatonin on the onset of puberty in male juvenile rats
- The Role of MicroRNAs in Vascular Diseases; Smooth Muscle Cell Differentiation and De-Differentiation
- The genes associated with gonadotropin-releasing hormone-dependent precocious puberty