Kosin Med J.
2024 Sep;39(3):195-200. 10.7180/kmj.24.124.
Satellite cell distribution in the medial rectus muscle in cadavers
- Affiliations
-
- 1Department of Ophthalmology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- KMID: 2559407
- DOI: http://doi.org/10.7180/kmj.24.124
Abstract
- Background
This study aimed to elucidate the potential usefulness of the medial rectus muscle of cadavers for research on satellite cells.
Methods
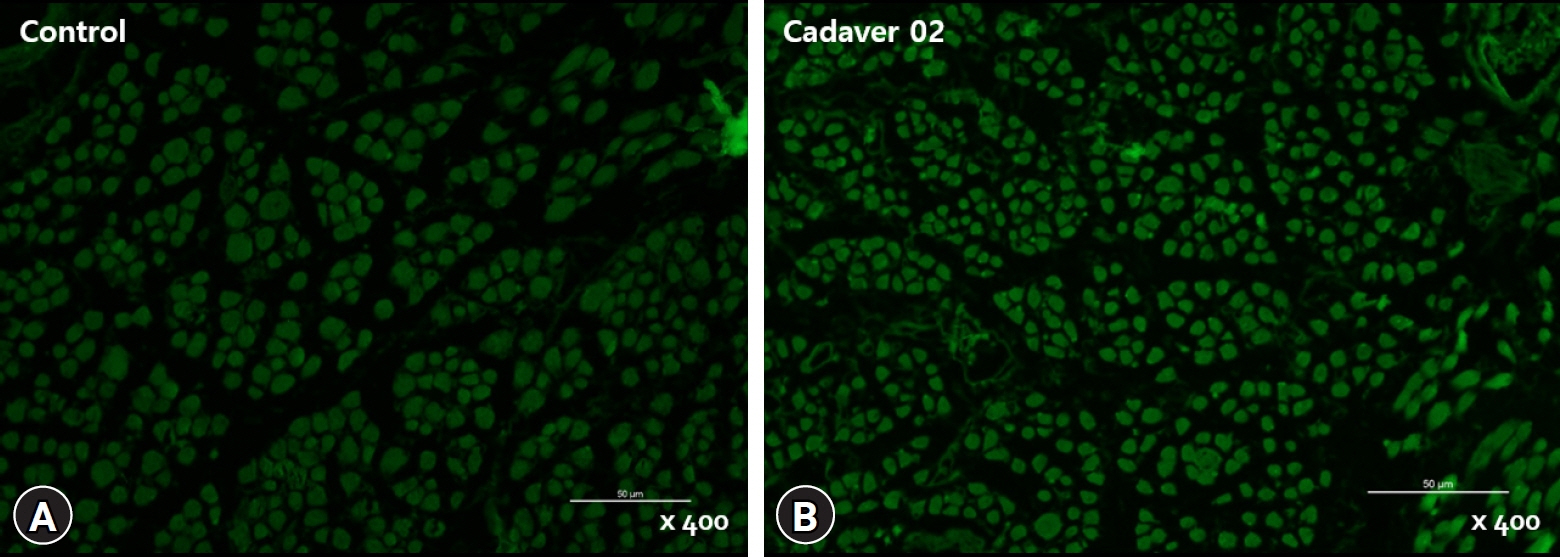
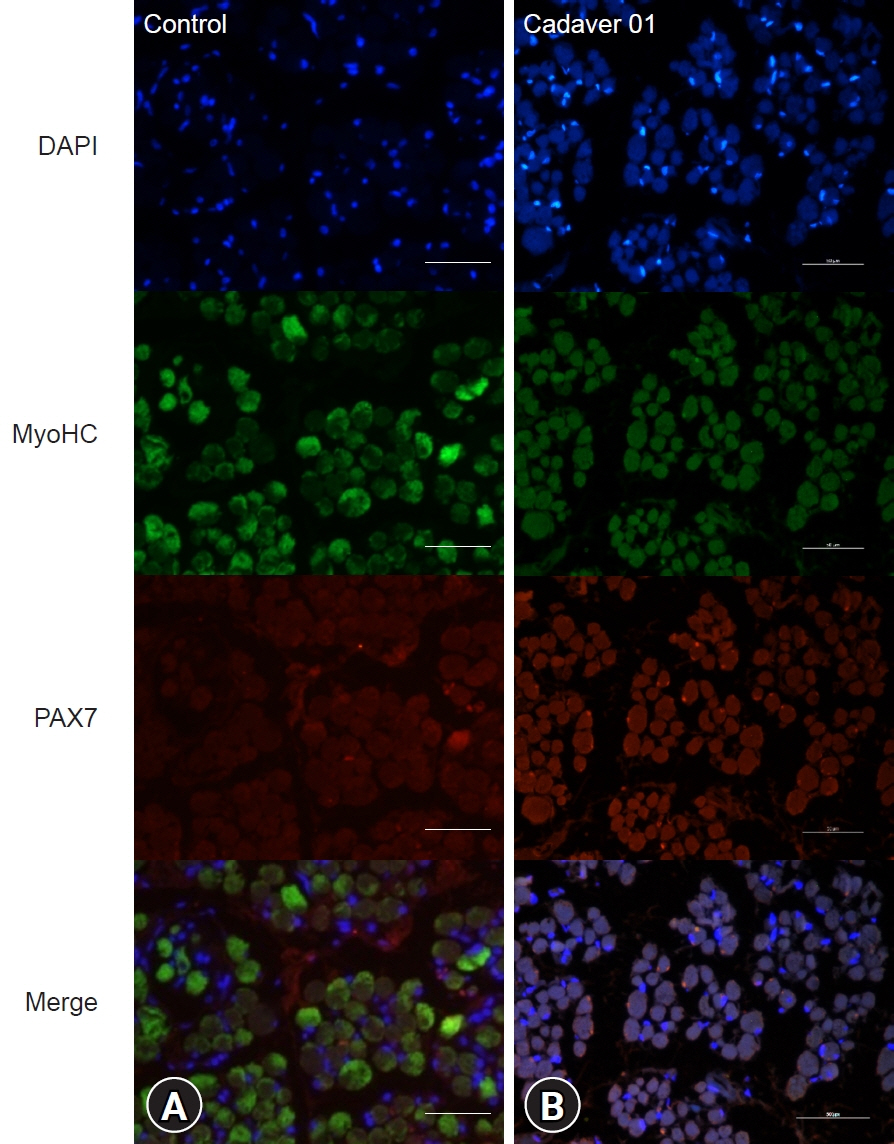
Twenty-four medial rectus muscles were obtained from 12 cadavers. The control group included six medial rectus muscles from three live adults without brain activity. The muscle fiber diameter and distribution of satellite cells were measured and compared. Immunohistochemistry for myosin heavy chain and the transcription factor PAX7 was performed, and the distributions of myocytes and satellite cells were evaluated.
Results
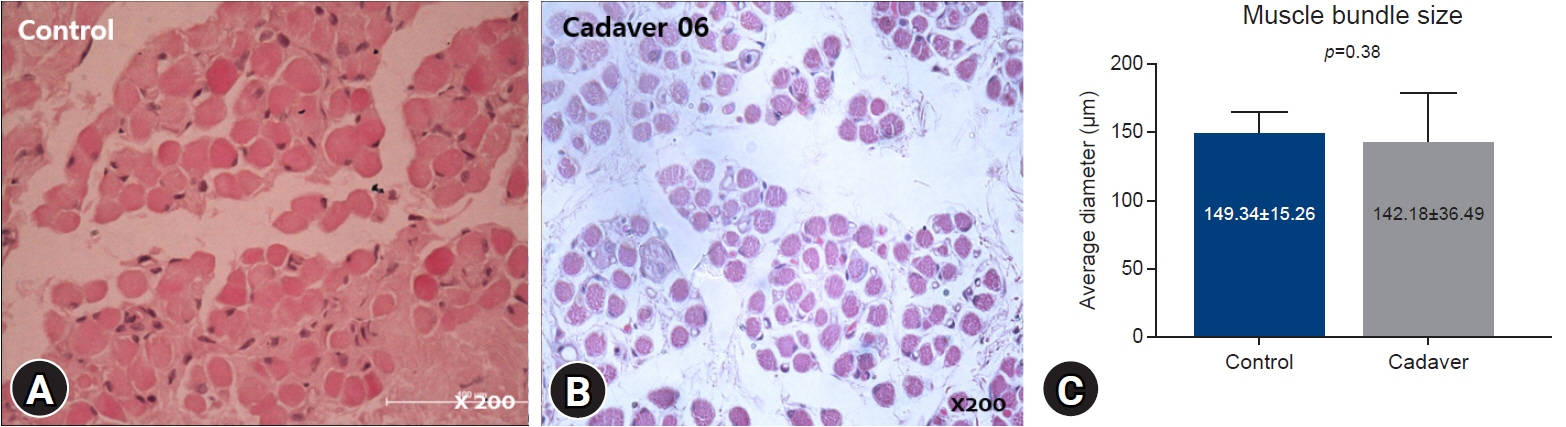
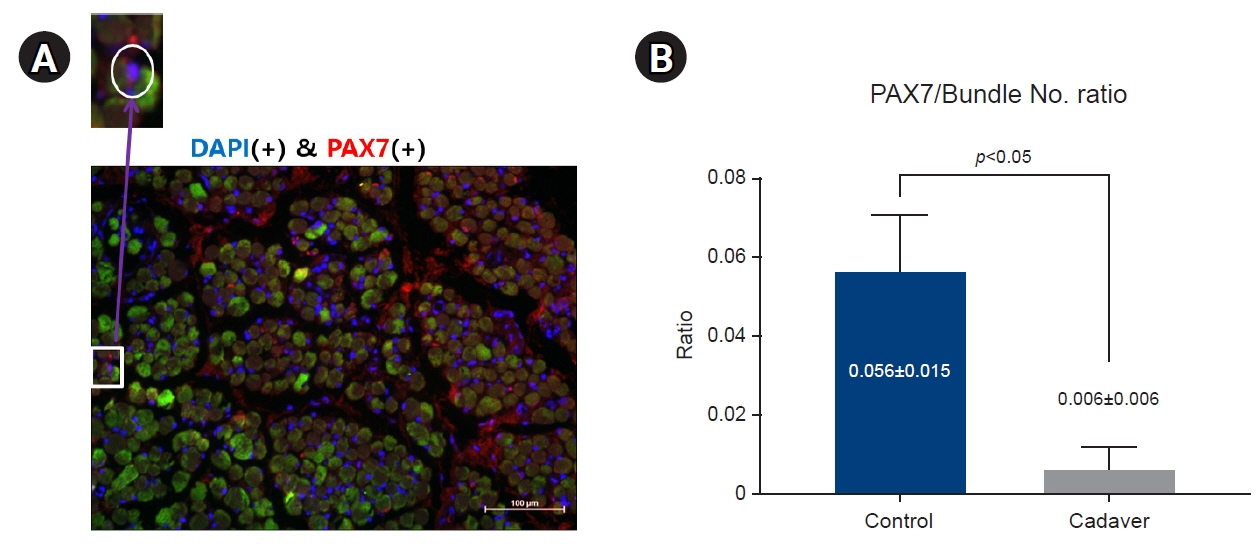
The average muscle fiber diameter was 142.18±36.49 μm in the cadaver group and 149.34±15.26 μm in the control group, and there was no significant difference between the two groups (p=0.38). The ratio of PAX7(+) cells to the number of muscle fibers was 0.056±0.015 in the control group and 0.006±0.006 in the cadaver group, reflecting a significant difference (p<0.05).
Conclusions
The medial rectus muscles of cadavers can be helpful in studying anatomical morphology; however, their usefulness in muscle satellite cell research appears to be limited.
Keyword
Figure
Reference
-
References
1. Boden SD, Labropoulos PA, McCowin P, Lestini WF, Hurwitz SR. Mechanical considerations for the syndesmosis screw. A cadaver study. J Bone Joint Surg Am. 1989; 71:1548–55.2. Brooks CH, Revell WJ, Heatley FW. Vascularity of the humeral head after proximal humeral fractures: an anatomical cadaver study. J Bone Joint Surg Br. 1993; 75:132–6.
Article3. Clarys JP, Provyn S, Marfell-Jones MJ. Cadaver studies and their impact on the understanding of human adiposity. Ergonomics. 2005; 48:1445–61.
Article4. Paik DJ, Shin SY. An anatomical study of the inferior oblique muscle: the embalmed cadaver vs the fresh cadaver. Am J Ophthalmol. 2009; 147:544–9.
Article5. Shin HJ, Lee SH, Ha TJ, Song WC, Koh KS. Intramuscular nerve distribution of the inferior oblique muscle. Curr Eye Res. 2020; 45:215–20.
Article6. Shin HJ, Lee SH, Ha TJ, Song WC, Lee AG, Koh KS. Intramuscular nerves of the inferior rectus muscle: distribution and characteristics. Curr Eye Res. 2020; 45:1598–603.7. Rae G, Newman WP, McGoey R, Donthamsetty S, Karpinski AC, Green J. The histopathologic reliability of tissue taken from cadavers within the gross anatomy laboratory. Anat Sci Educ. 2018; 11:207–14.
Article8. Wood A, Whiten S, Mcvee J, Issberner J, Jackson D, Herrington CS. Histopathology from the dissecting room: are cadavers a suitable source of educationally useful histopathology specimens? Anatomy. 2015; 9:26–33.
Article9. Novak JS, Mazala DA, Nearing M, Hindupur R, Uapinyoying P, Habib NF, et al. Human muscle stem cells are refractory to aging. Aging Cell. 2021; 20:e13411.
Article10. Kang G, Kim SE. How to write an original article in medicine and medical science. Kosin Med J. 2022; 37:96–101.
Article11. Lee HS. Ethical issues in clinical research and publication. Kosin Med J. 2022; 37:278–82.12. Latil M, Rocheteau P, Chatre L, Sanulli S, Memet S, Ricchetti M, et al. Skeletal muscle stem cells adopt a dormant cell state post mortem and retain regenerative capacity. Nat Commun. 2012; 3:903.
Article13. Lindstrom M, Tjust AE, Pedrosa Domellof F. Pax7-positive cells/satellite cells in human extraocular muscles. Invest Ophthalmol Vis Sci. 2015; 56:6132–43.
Article14. McLoon LK, Wirtschafter J. Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest Ophthalmol Vis Sci. 2003; 44:1927–32.
Article15. Kim CZ, Lee SJ. Increased myofiber size and reduced satellite cell numbers in medial rectus muscle of patients with intermittent exotropia. Strabismus. 2020; 28:201–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microanatomy of the Artery of the External Ocular Rectus Muscles: II. Arterial Distribution of the Rectus Muscles
- Surgical anatomy of transversus abdominis muscle for transversus abdominis release
- Morphology and Distribution of Motorneurons of Oculomotor Nucleus and Medial Longitudinal Fasciculus in the Cat
- Comparison of the Change in Tension of Lateral Rectus Muscle between Bilateral Recession of Lateral Rectus Muscle and Unilateral Recession of Lateral Rectus Muscle and Resection of Medial Rectus Muscle in Rabbit
- Medial transposition of the lateral rectus muscle in experimentally induced medial rectus paralysis