Korean Circ J.
2024 Sep;54(9):549-561. 10.4070/kcj.2023.0241.
Cardiovascular Outcomes of Sodium-Glucose Cotransporter-2 Inhibitors Therapy in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Systematic Review and Updated Meta-Analysis
- Affiliations
-
- 1Federal University of Campina Grande, Campina Grande, PB, Brazil
- 2University of the Region of Joinville, Joinville, SC, Brazil
- 3Bahiana School of Medicine and Public Health, Salvador, BA, Brazil
- 4University of the Extreme South of Santa Catarina, Criciúma, SC, Brazil
- 5Cardiff University School of Medicine, Cardiff, UK
- 6University of Brasília, Brasília, DF, Brazil
- 7Katz Family Division of Nephrology and Hypertension, Department of Medicine, University of Miami, Miami, FL, USA
- 8Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
- KMID: 2559127
- DOI: http://doi.org/10.4070/kcj.2023.0241
Abstract
- Background and Objectives
The efficacy of sodium-glucose cotransporter-2 inhibitors (SGLT2i) may depend on renal function, and this raises theoretical concern over its effects on cardiovascular outcomes in patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD).
Methods
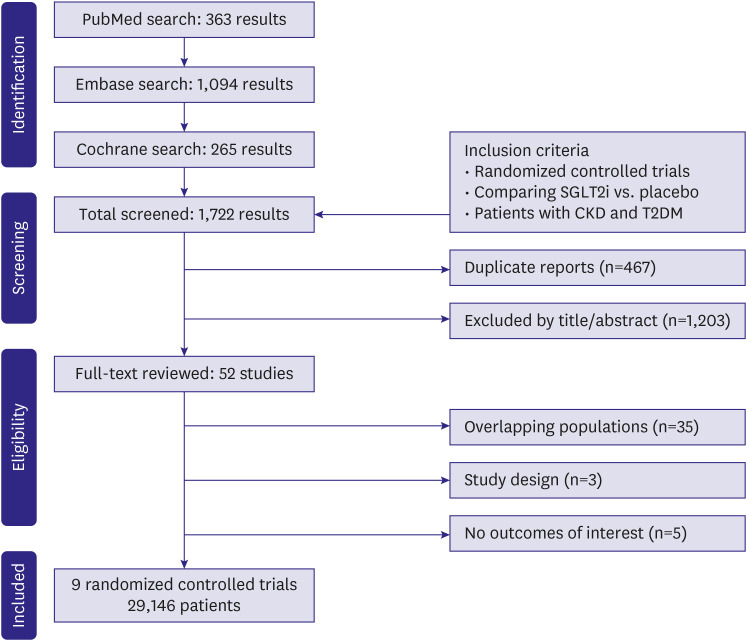
This systematic review and updated meta-analysis of randomized controlled trials (RCTs) compared cardiovascular outcomes of patients with T2DM and CKD treated with SGLT2i to placebo. PubMed, Embase, and Cochrane were systematically searched. Prespecified subgroup analyses were performed in strata of estimated glomerular filtration rate (eGFR) of <45 mL/min/1.73 m2 and 45 to 59 mL/min/1.73 m2 .
Results
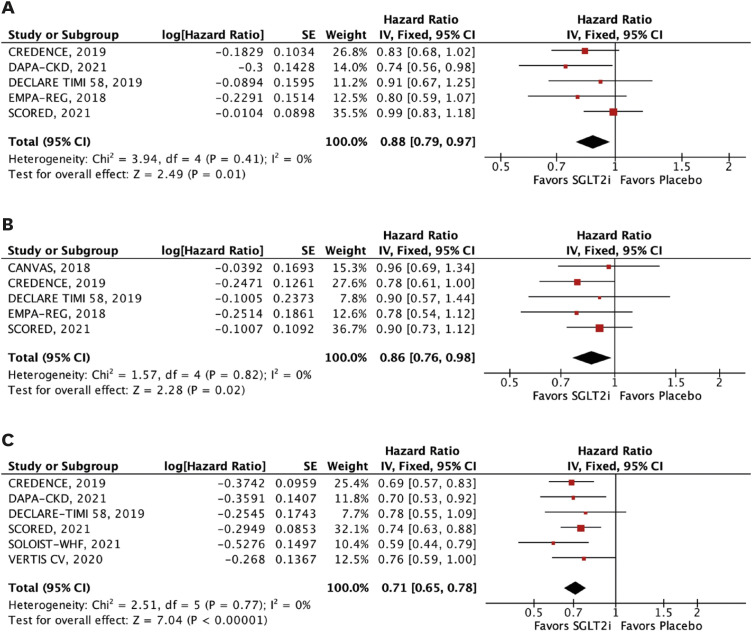
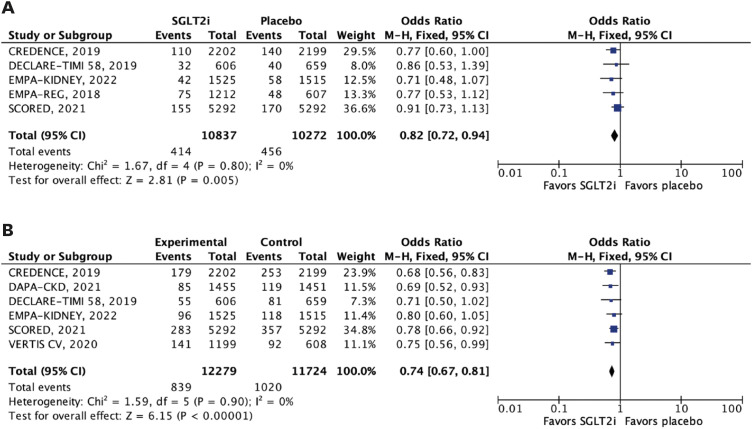
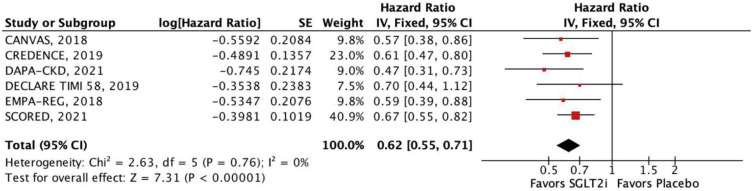
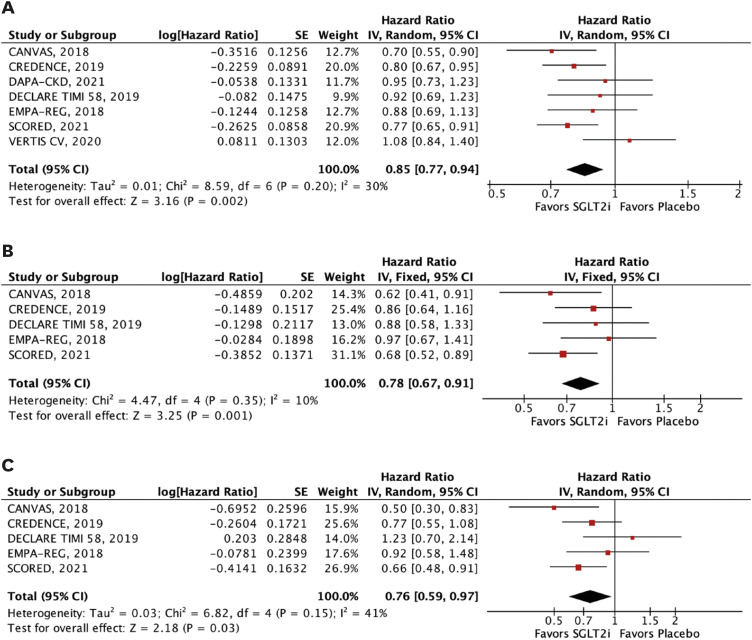
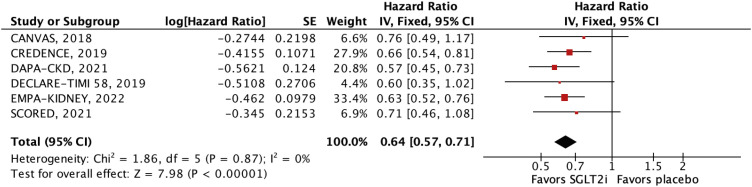
Nine RCTs comprising 29,146 patients were selected. Average follow-up ranged from 0.75 to 4.2 years. SGLT2i were shown to reduce the risk of all-cause mortality (hazard ratio [HR], 0.88; 95% confidence interval [CI], 0.79–0.97; p=0.01), the composite of cardiovascular mortality or hospitalizations for heart failure (HHF: HR, 0.71; 95% CI, 0.65–0.78; p<0.001), cardiovascular mortality (HR, 0.86; 95% CI, 0.76–0.98; p=0.02), HHF (HR, 0.62; 95% CI, 0.55–0.71; p<0.001), major adverse cardiovascular events (HR, 0.85; 95% CI, 0.77–0.94; p=0.002), stroke (HR, 0.76; 95% CI, 0.59–0.97; p=0.03), and myocardial infarction (HR, 0.78; 95% CI, 0.67–0.91; p=0.001). These findings were consistent over strata of eGFR, albeit with a lower incidence of stroke in patients treated with SGLT2i with eGFR <45 mL/min/1.73 m2 (p-value for interaction=0.04).
Conclusions
Compared with a placebo, patients with T2DM and CKD treated with SGLT2i experience a reduction in all-cause mortality, cardiovascular mortality, and HHF.
Keyword
Figure
Reference
-
1. Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008; 88:1254–1264. PMID: 18801858.2. Khan MA, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020; 10:107–111. PMID: 32175717.3. Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016; 12:73–81. PMID: 26553517.4. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021; 143:1157–1172. PMID: 33720773.5. Jardine MJ, Zhou Z, Mahaffey KW, et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol. 2020; 31:1128–1139. PMID: 32354987.6. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–357. PMID: 30415602.7. The EMPA-KIDNEY Collaborative Group. Herrington WG, Staplin N, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023; 388:117–127. PMID: 36331190.8. Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018; 137:119–129. PMID: 28904068.9. Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021; 384:129–139. PMID: 33200891.10. Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020; 383:1425–1435. PMID: 32966714.11. Petrykiv S, Sjöström CD, Greasley PJ, Xu J, Persson F, Heerspink HJ. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol. 2017; 12:751–759. PMID: 28302903.12. Toyama T, Neuen BL, Jun M, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab. 2019; 21:1237–1250. PMID: 30697905.13. Salah HM, Al’Aref SJ, Khan MS, et al. Effects of sodium-glucose cotransporter 1 and 2 inhibitors on cardiovascular and kidney outcomes in type 2 diabetes: a meta-analysis update. Am Heart J. 2021; 233:86–91. PMID: 33385359.14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71. PMID: 33782057.15. Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZ. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017; 136:1643–1658. PMID: 29061576.16. Zhang Y, Han Q. A review of cardiovascular benefits of SGLT2 inhibitors. Medicine (Baltimore). 2022; 101:e30310. PMID: 36086785.17. Solomon SD, Lin J, Solomon CG, et al. Influence of albuminuria on cardiovascular risk in patients with stable coronary artery disease. Circulation. 2007; 116:2687–2693. PMID: 18025537.18. Heerspink HJ, Kosiborod M, Inzucchi SE, Cherney DZ. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018; 94:26–39. PMID: 29735306.19. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016; 134:752–772. PMID: 27470878.20. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021; 384:117–128. PMID: 33200892.21. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–39. PMID: 30424892.22. Tsai WH, Chuang SM, Liu SC, et al. Effects of SGLT2 inhibitors on stroke and its subtypes in patients with type 2 diabetes: a systematic review and meta-analysis. Sci Rep. 2021; 11:15364. PMID: 34321571.23. Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011; 108:56–62. PMID: 21529739.24. Zelniker TA, Bonaca MP, Furtado RH, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020; 141:1227–1234. PMID: 31983236.25. Barreto J, Campos-Staffico AM, Nadruz W, Quinaglia T, Sposito AC. The role of SGLT2i in attenuating residual cardiovascular risk through blood pressure-lowering: mechanistic insights and perspectives. Front Clin Diabetes Healthc. 2023; 4:1243530. PMID: 37822556.26. Hsieh CY, Sung SF. From kidney protection to stroke prevention: the potential role of sodium glucose cotransporter-2 inhibitors. Int J Mol Sci. 2022; 24:351. PMID: 36613795.27. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022; 102:S1–S127. PMID: 36272764.28. Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018; 138:1537–1550. PMID: 29941478.29. Persson F, Rossing P, Vart P, et al. Efficacy and safety of dapagliflozin by baseline glycemic status: a prespecified analysis from the DAPA-CKD trial. Diabetes Care. 2021; 44:1894–1897. PMID: 34183431.30. Austin PC, Steyerberg EW. The number of subjects per variable required in linear regression analyses. J Clin Epidemiol. 2015; 68:627–636. PMID: 25704724.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glucose Lowering Effect of SGLT2 Inhibitors: A Review of Clinical Studies
- Emerging Safety Issues of Dipeptidyl Peptidase-4 Inhibitors and Sodium Glucose Cotransporter 2 Inhibitors: How to Interpret and Apply in Clinical Practice
- New anti-diabetic agents
- Prevention of Major Adverse Cardiovascular and Renal Outcomes with Sodium-Glucose Cotransporter 2 Inhibitors
- Sodium-Glucose Cotransporter 2 Inhibitors for People with Type 1 Diabetes