Endocrinol Metab.
2024 Aug;39(4):653-658. 10.3803/EnM.2024.1919.
Ketonuria as an Indicator of Improvement of Renal Function in Patients with Type 2 Diabetes Receiving SGLT2 Inhibitor Treatment
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 4Veterans Medical Research Institute, Veterans Health Service Medical Center, Seoul, Korea
- KMID: 2558949
- DOI: http://doi.org/10.3803/EnM.2024.1919
Abstract
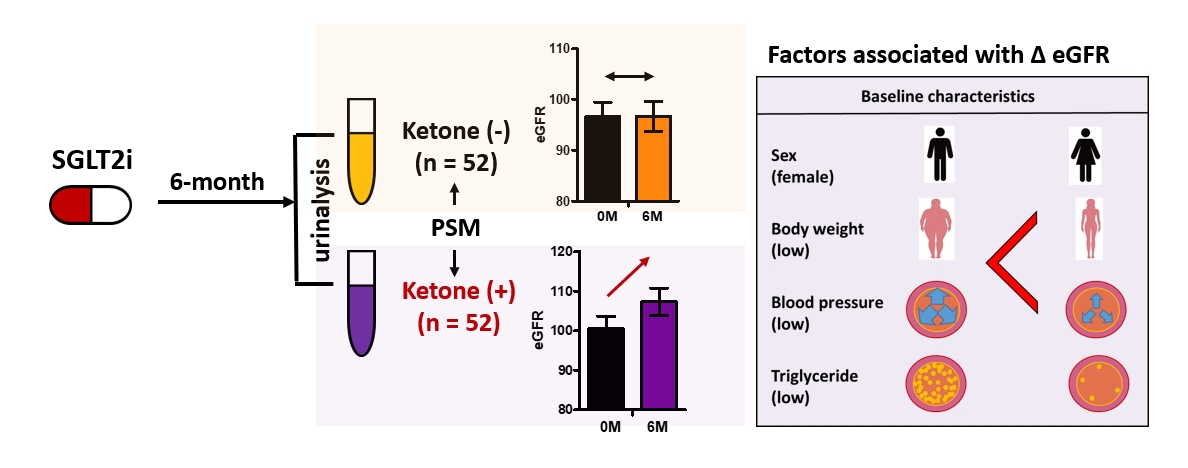
- We investigated the potential association between ketonuria during treatment with sodium-glucose cotransporter-2 (SGLT2) inhibitors and its renoprotective effect in patients with type 2 diabetes. We included 192 patients who had received SGLT2 inhibitors for more than 6 months. After propensity score matching, 52 patients each were allocated into groups with or without ketonuria, respectively. The estimated glomerular filtration rate exhibited a significant improvement only in subjects with ketonuria (without ketonuria: mean difference, –0.02 mL/min/1.73 m2 [95% confidence interval (CI), –3.87 to 3.83 mL/min/1.73 m2] vs. with ketonuria: mean difference, 6.81 mL/min/1.73 m2 [95% CI, 3.16 to 10.46 mL/min/1.73 m2]; P<0.001). Improvement in estimated glomerular filtration rate at 6 months was associated with female sex and lower baseline body weight, blood pressure, and triglyceride levels in patients with ketonuria. In conclusion, the presence of ketonuria was associated with the renoprotective effect of SGLT2 inhibitors, and female sex and the absence of metabolic syndrome components may serve as additional indicators of these medications’ substantial renoprotective effects in individuals with ketonuria.
Keyword
Figure
Reference
-
1. Ghaderian SB, Hayati F, Shayanpour S, Beladi Mousavi SS. Diabetes and end-stage renal disease; a review article on new concepts. J Renal Inj Prev. 2015; 4:28–33.2. Kravets I, Mallipattu SK. The role of podocytes and podocyte-associated biomarkers in diagnosis and treatment of diabetic kidney disease. J Endocr Soc. 2020; 4:bvaa029.
Article3. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023; 388:117–27.
Article4. Heerspink HJ, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383:1436–46.
Article5. Min SH, Oh TJ, Baek SI, Lee DH, Kim KM, Moon JH, et al. Degree of ketonaemia and its association with insulin resistance after dapagliflozin treatment in type 2 diabetes. Diabetes Metab. 2018; 44:73–6.
Article6. Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014; 25:42–52.
Article7. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999; 15:412–26.
Article8. Fang Y, Chen B, Gong AY, Malhotra DK, Gupta R, Dworkin LD, et al. The ketone body β-hydroxybutyrate mitigates the senescence response of glomerular podocytes to diabetic insults. Kidney Int. 2021; 100:1037–53.
Article9. Rojas-Morales P, Pedraza-Chaverri J, Tapia E. Ketone bodies for kidney injury and disease. Adv Redox Res. 2021; 2:100009.
Article10. Tomita I, Kume S, Sugahara S, Osawa N, Yamahara K, Yasuda-Yamahara M, et al. SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced mTORC1 inhibition. Cell Metab. 2020; 32:404–19.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitor
- Sodium-Glucose Cotransporter-2 Inhibitor for Renal Function Preservation in Patients with Type 2 Diabetes Mellitus: A Korean Diabetes Association and Korean Society of Nephrology Consensus Statement
- Sodium-glucose cotransporter-2 inhibitor for renal function preservation in patients with type 2 diabetes mellitus: a Korean Diabetes Association and Korean Society of Nephrology consensus statement
- Glucose Lowering Effect of SGLT2 Inhibitors: A Review of Clinical Studies
- Evaluation of the Clinical Significance of Ketonuria