Endocrinol Metab.
2024 Aug;39(4):579-589. 10.3803/EnM.2024.1918.
Dynamic Risk Model for the Medical Treatment of Graves’ Hyperthyroidism according to Treatment Duration
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2558942
- DOI: http://doi.org/10.3803/EnM.2024.1918
Abstract
- Background
Changes in thyrotropin receptor antibody (TRAb) levels are associated with the clinical outcomes of Graves’ hyperthyroidism. However, the effects of the patterns of TRAb changes on patient prognosis according to the treatment duration of antithyroid drugs (ATDs) are not well established.
Methods
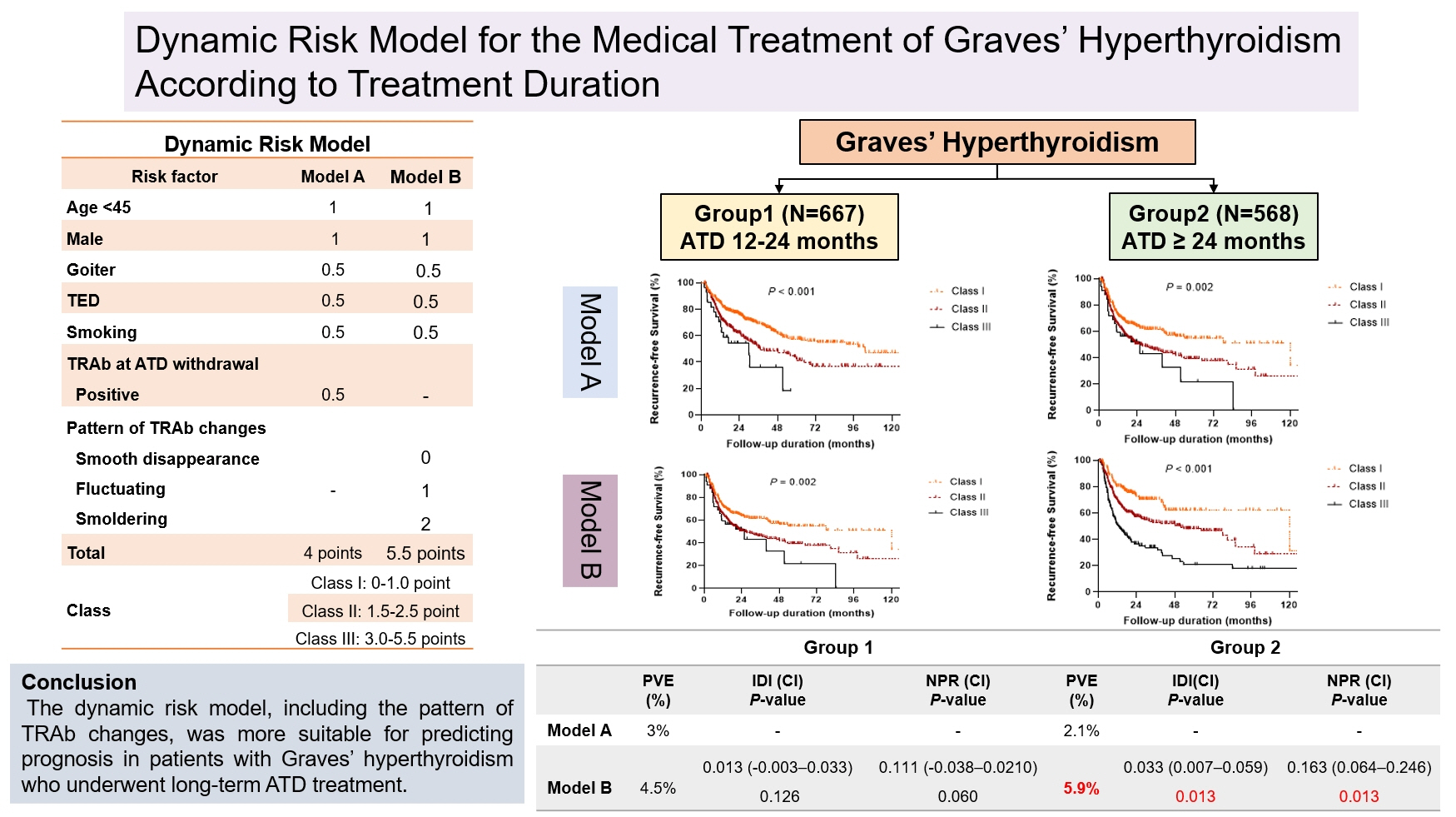
In this retrospective cohort study, 1,235 patients with Graves’ hyperthyroidism who were treated with ATDs for more than 12 months were included. Patients were divided into two groups according to treatment duration: group 1 (12–24 months) and group 2 (>24 months). Risk prediction models comprising age, sex, and either TRAb levels at ATD withdrawal (model A) or patterns of TRAb changes (model B) were compared.
Results
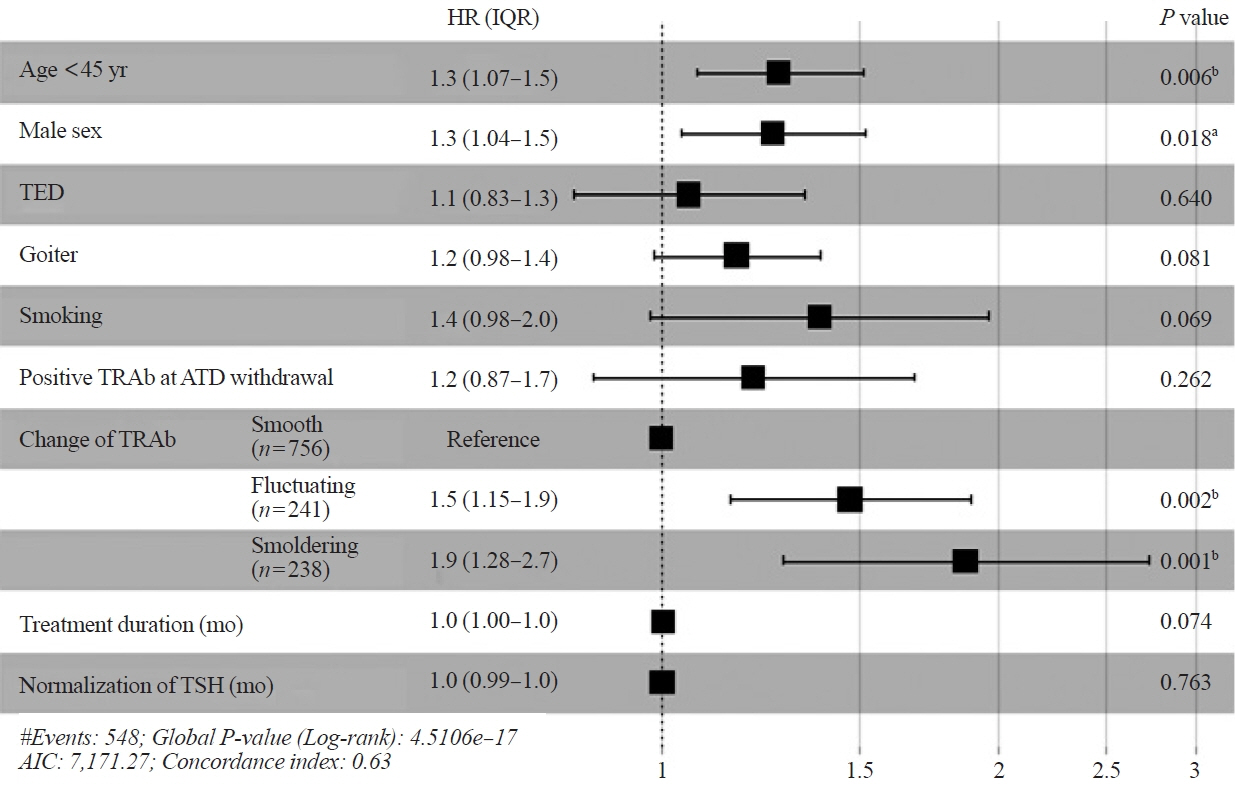
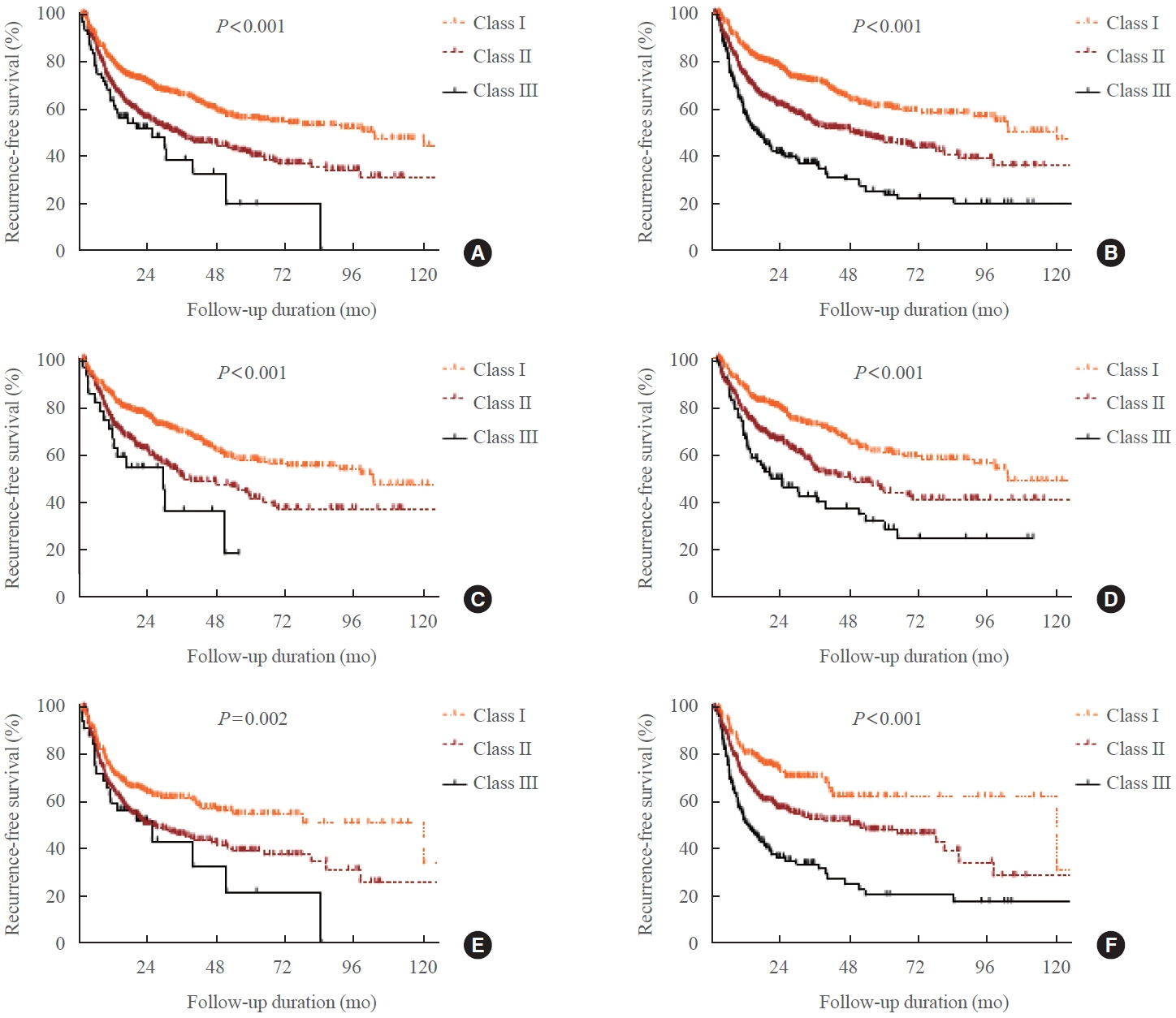
The median treatment duration in groups 1 (n=667, 54%) and 2 (n=568, 46%) was 17.3 and 37.1 months, respectively. The recurrence rate was significantly higher in group 2 (47.9%) than in group 1 (41.4%, P=0.025). Group 2 had significantly more goiter, thyroid eye disease, and fluctuating and smoldering type of TRAb pattern compared with group 1 (all P<0.001). The patterns of TRAb changes were an independent risk factor for recurrence after adjusting for other confounding factors in all patients, except in group 1. Integrated discrimination improvement and net reclassification improvement analyses showed that model B performed better than model A in all patients, except in group 1.
Conclusion
The dynamic risk model, including the patterns of TRAb changes, was more suitable for predicting prognosis in patients with Graves’ hyperthyroidism who underwent longer ATD treatment duration.
Keyword
Figure
Reference
-
1. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016; 26:1343–421.
Article2. Kahaly GJ, Bartalena L, Hegedus L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association guideline for the management of Graves’ hyperthyroidism. Eur Thyroid J. 2018; 7:167–86.
Article3. Abraham P, Avenell A, Park CM, Watson WA, Bevan JS. A systematic review of drug therapy for Graves’ hyperthyroidism. Eur J Endocrinol. 2005; 153:489–98.
Article4. Azizi F, Amouzegar A, Tohidi M, Hedayati M, Khalili D, Cheraghi L, et al. Increased remission rates after long-term methimazole therapy in patients with Graves’ disease: results of a randomized clinical trial. Thyroid. 2019; 29:1192–200.
Article5. Azizi F, Malboosbaf R. Long-term antithyroid drug treatment: a systematic review and meta-analysis. Thyroid. 2017; 27:1223–31.
Article6. Park SY, Kim BH, Kim M, Hong AR, Park J, Park H, et al. The longer the antithyroid drug is used, the lower the relapse rate in Graves’ disease: a retrospective multicenter cohort study in Korea. Endocrine. 2021; 74:120–7.
Article7. Jin M, Jang A, Kim CA, Kim TY, Kim WB, Shong YK, et al. Long-term follow-up result of antithyroid drug treatment of Graves’ hyperthyroidism in a large cohort. Eur Thyroid J. 2023; 12:e220226.
Article8. Chung JH. Antithyroid drug treatment in Graves’ disease. Endocrinol Metab (Seoul). 2021; 36:491–9.
Article9. Bandai S, Okamura K, Fujikawa M, Sato K, Ikenoue H, Kitazono T. The long-term follow-up of patients with thionamide-treated Graves’ hyperthyroidism. Endocr J. 2019; 66:535–45.10. Cooper DS. Long-term antithyroid drug treatment of patients with graves’ disease. Clin Thyroidol. 2019; 31:230–3.
Article11. Yu J, Baek HS, Jeong C, Jo K, Lee J, Ha J, et al. The early changes in thyroid-stimulating immunoglobulin bioassay over anti-thyroid drug treatment could predict prognosis of Graves’ disease. Endocrinol Metab (Seoul). 2023; 38:338–46.
Article12. Vos XG, Endert E, Zwinderman AH, Tijssen JG, Wiersinga WM. Predicting the risk of recurrence before the start of antithyroid drug therapy in patients with Graves’ hyperthyroidism. J Clin Endocrinol Metab. 2016; 101:1381–9.
Article13. World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed. Geneva: WHO;2007.14. Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the European group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid. 2008; 18:333–46.
Article15. Vaidya B, Wright A, Shuttleworth J, Donohoe M, Warren R, Brooke A, et al. Block & replace regime versus titration regime of antithyroid drugs for the treatment of Graves’ disease: a retrospective observational study. Clin Endocrinol (Oxf). 2014; 81:610–3.16. Park S, Song E, Oh HS, Kim M, Jeon MJ, Kim WG, et al. When should antithyroid drug therapy to reduce the relapse rate of hyperthyroidism in Graves’ disease be discontinued? Endocrine. 2019; 65:348–56.
Article17. Kwon H, Kim WG, Jang EK, Kim M, Park S, Jeon MJ, et al. Usefulness of measuring thyroid stimulating antibody at the time of antithyroid drug withdrawal for predicting relapse of Graves disease. Endocrinol Metab (Seoul). 2016; 31:300–10.
Article18. Konishi T, Okamoto Y, Ueda M, Fukuda Y, Harusato I, Tsukamoto Y, et al. Drug discontinuation after treatment with minimum maintenance dose of an antithyroid drug in Graves’ disease: a retrospective study on effects of treatment duration with minimum maintenance dose on lasting remission. Endocr J. 2011; 58:95–100.
Article19. Jeon MJ, Kim WG, Jang EK, Choi YM, Lee YM, Sung TY, et al. Thyroglobulin level in fine-needle aspirates for preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma: two different cutoff values according to serum thyroglobulin level. Thyroid. 2015; 25:410–6.
Article20. Ahn HY, Cho SW, Lee MY, Park YJ, Koo BS, Chang HS, et al. Prevalence, treatment status, and comorbidities of hyperthyroidism in Korea from 2003 to 2018: a nationwide population study. Endocrinol Metab (Seoul). 2023; 38:436–44.
Article21. Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves’ disease. J Clin Endocrinol Metab. 2012; 97:4549–58.
Article22. Brito JP, Payne S, Singh Ospina N, Rodriguez-Gutierrez R, Maraka S, Sangaralingham LR, et al. Patterns of use, efficacy, and safety of treatment options for patients with Graves’ disease: a nationwide population-based study. Thyroid. 2020; 30:357–64.
Article23. Baek HS, Lee J, Jeong CH, Lee J, Ha J, Jo K, et al. The prediction model using thyroid-stimulating immunoglobulin bioassay for relapse of Graves’ disease. J Endocr Soc. 2022; 6:bvac023.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Diagnosis and Management of Hyperthyroidism in Korea: Consensus Report of the Korean Thyroid Association
- The treatment of Graves' disease in children and adolescents
- Diagnosis and Treatment of Hyperthyroidism in Pregnancy
- Spinal anesthesia for urgent Cesarean section in a patient with uncontrolled hyperthyroidism due to Graves’ disease - A case report -
- Massive cerebral venous sinus thrombosis secondary to Graves' disease