J Korean Neurosurg Soc.
2024 Sep;67(5):521-530. 10.3340/jkns.2023.0219.
Investigation of Neuroprotective Efficacy of Dexpanthenol in an Experimental Head Injury Model
- Affiliations
-
- 1Brain and Nerve Surgery Clinic, Ministry of Health Necip Fazıl City Hospital, Kahramanmaraş, Turkey
- 2Brain and Nerve Surgery Clinic, Ministry of Health Karadeniz Ereğli State Hospital, Zonguldak, Turkey
- 3Department of Neurosurgery, Inonu University Faculty of Medicine, Malatya, Turkey
- KMID: 2558677
- DOI: http://doi.org/10.3340/jkns.2023.0219
Abstract
Objective
: Dexpanthenol (DXP), which has known neuroprotective effects, has been shown to be beneficial in various experimental models and ischaemic diseases. The aim of this study was to investigate the possible neuroprotective effects of DXP in a traumatic brain injury (TBI) model.
Methods
: Thirty-six Wistar-Albino female rats, approximately 6 months old, weighing 220–285 g were used. All rats were subjected to closed head trauma by dropping a weight of 350 g on the parietal region from a height of 50 cm at an angle of 180 degrees in the prepared head trauma model setup. The rats were divided into four groups as control (group 1), trauma (group 2), trauma + DXP (group 3), and DXP (group 4). In group 3, DXP was administered intraperitoneally at a dose of 500 mg/kg for six times at 30 minutes, 6, 12, 24, 36, and 48 hours. In group 4, DXP was administered intraperitoneally simultaneously with group 3 without causing head trauma. Blood samples were taken from all rats 72 hours later for biochemical examination. After blood samples were taken, rats were decapitated under general anaesthesia. Cerebral tissue samples were taken from decapitated rats for immunohistochemical and histopathological examination.
Results
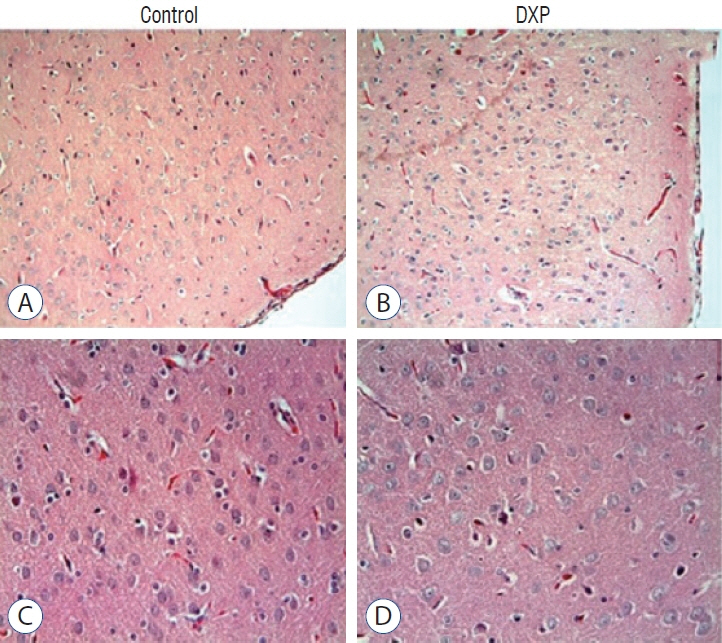
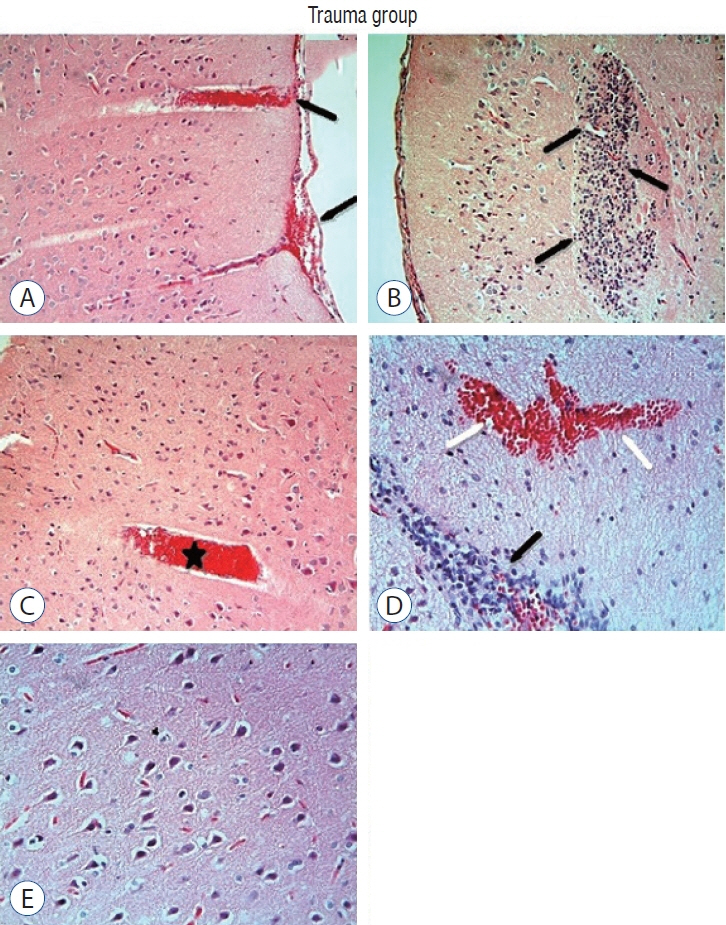
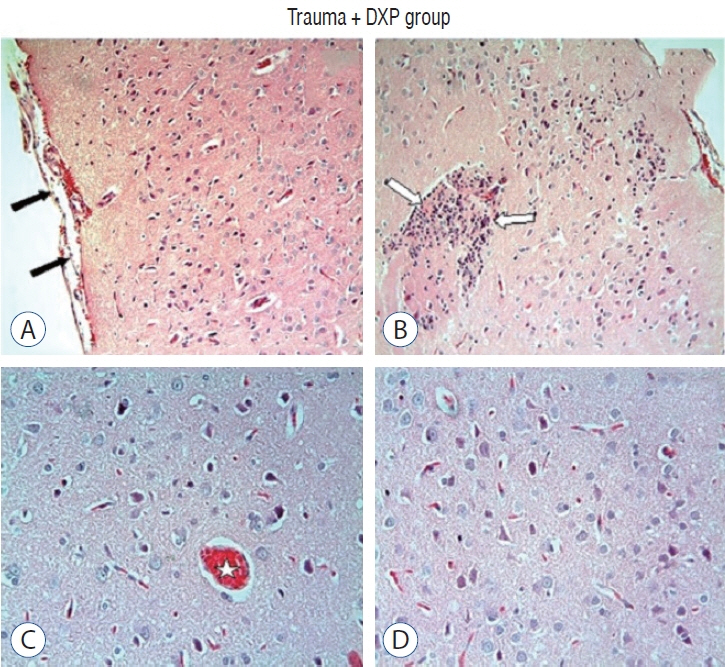
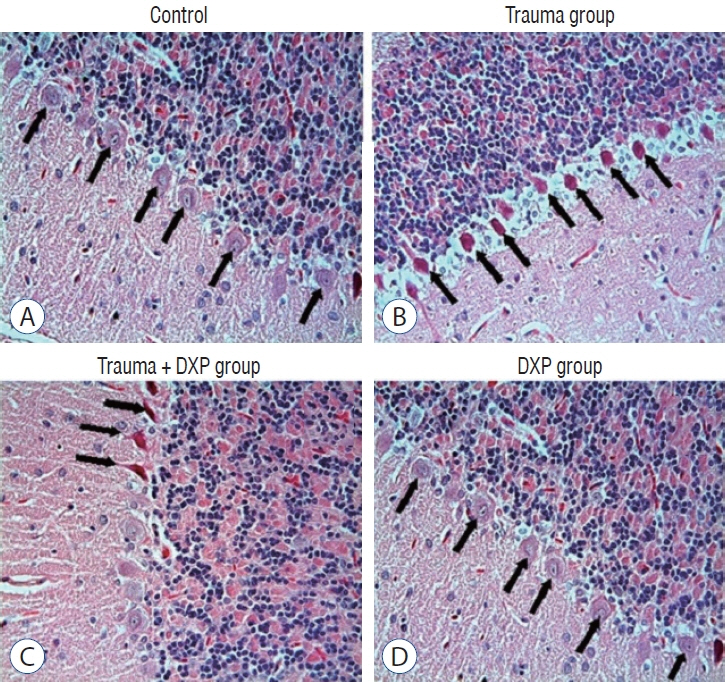
: Cytokine markers were found to be increased in posttraumatic brain tissue. Malondialdehyde and glutathione reductase levels were lower in group 3 compared to group 2. In addition, superoxide dismutase, glutathione peroxidase and catalase levels were significantly higher in group 3 compared to group 2. In histological evaluation, congestion in the piamater layer, cell infiltration, vascular congestion, hemorrhage and neuronal degeneration were significantly decreased in group 3 compared to group 2. DXP seems to be beneficial in neurological recovery in terms of histological and oxidative changes after head trauma in rats.
Conclusion
: DXP should be further evaluated for its possible therapeutic effect in TBI.
Figure
Reference
-
References
1. Aebi H. Catalase in vitro. Methods Enzymol. 105:121–126. 1984.2. Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 5:1027–1039. discussion 1039-1040. 1999.
Article3. Beynon C, Hertle DN, Unterberg AW, Sakowitz OW. Clinical review: traumatic brain injury in patients receiving antiplatelet medication. Crit Care. 16:228. 2012.
Article4. Binder S, Corrigan JD, Langlois JA. The public health approach to traumatic brain injury: an overview of CDC’s research and programs. J Head Trauma Rehabil. 20:189–195. 2005.5. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 82:70–77. 1959.6. Erdogan MA, Yigitturk G, Erbas O, Taskıran D. Neuroprotective effects of dexpanthenol on streptozotocin-induced neuronal damage in rats. Drug Chem Toxicol. 5:2160–2168. 2022.
Article7. Gean AD, Fischbein NJ. Head trauma. Neuroimaging Clin N Am. 4:527–556. 2010.
Article8. Guerrero JL, Thurman DJ, Sniezek JE. Emergency department visits associated with traumatic brain injury: United States, 1995-1996. Brain Inj. 14:181–186. 2000.
Article9. Gülmez A, Kuru Bektaşoğlu P, Tönge Ç, Yaprak A, Türkoğlu ME, Önder E, et al. Neuroprotective effects of dexpanthenol on rabbit spinal cord ischemia/reperfusion injury model. World Neurosurg. 167:e172–e183. 2022.
Article10. Hall ED, Braughler JM. Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med. 6:303–13. 1989.11. Karahan G, Kaya H, Eyceyurt RS, Erdogan MA, Yigitturk G, Erbas O. Dexpanthenol reduces fibrosis and aids repair following nerve laceration and neurorrhaphy. Exp Ther Med. 3:207. 2021.
Article12. Khalili H, Rakhsha A, Ghaedian T, Niakan A, Masoudi N. Application of brain perfusion SPECT in the evaluation of response to zolpidem therapy in consciousness disorder due to traumatic brain injury. Indian J Nucl Med. 4:315–320. 2020.
Article13. Li M, Ona VO, Chen M, Kaul M, Tenneti L, Zhang X, et al. Functional role and therapeutic implications of neuronal caspase-1 and -3 in a mouse model of traumatic spinal cord injury. Neuroscience. 99:333–342. 2000.
Article14. Liu Y, Yang H, Jia G, Li L, Chen H, Bi J, et al. The synergistic neuroprotective effects of combined rosuvastatin and resveratrol pretreatment against cerebral ischemia/reperfusion injury. J Stroke Cerebrovasc Dis. 27:1697–1704. 2018.
Article15. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1:265–275. 1951.16. Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7:728–741. 2008.
Article17. MacLaughlin BW, Plurad DS, Sheppard W, Bricker S, Bongard F, Neville A, et al. The impact of intracranial pressure monitoring on mortality after severe traumatic brain injury. Am J Surg. 210:1082–1086. discussion 1086-1087. 2015.
Article18. Marmarou A. Traumatic brain edema: an overview. Acta Neurochir Suppl (Wien). 60:421–424. 1994.
Article19. Marmarou A, Foda MA, van den Brink W, Campbell J, Kita H, Demetriadou K. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg. 80:291–300. 1994.20. Masomi-Bornwassser J, Freguia F, Müller-Werkmeister H, Kempski O, Giese A, Keric N. Effect of irrigation on fibrinolytic rtPA therapy in a clot model of intracerebral haemorrhage: a systematic in vitro study. Acta Neurochir (Wien). 160:1159–1165. 2018.21. Masson F, Thicoipe M, Aye P, Mokni T, Senjean P, Schmitt V, et al. Epidemiology of severe brain injuries: a prospective population-based study. J Trauma. 51:481–489. 2001.
Article22. Miller JD, Piper IR, Jones PA : Pathophysiology of head injury in Narayan RK, Wilberger JE, Povlishock JT (eds) : Neorotrauma. New York : McGrawHill, 1996.23. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–358. 1979.
Article24. Ozdamar Unal G, Asci H, Erzurumlu Y, Ilhan I, Hasseyid N, Ozmen O. Dexpanthenol may protect the brain against lipopolysaccharide induced neuroinflammation via anti-oxidant action and regulating CREB/BDNF signaling. Immunopharmacol Immunotoxicol. 44:186–193. 2022.
Article25. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 70:158–169. 1967.26. Prasad GL. Steroids for delayed cerebral edema after traumatic brain injury. Surg Neurol Int. 12:46. 2021.27. Prisk D. Anaesthesia of the head trauma patient in BSAVA Congress Proceedings 2019. Birmingham: BSAVA Library;2019. p. 255–256.28. Sogut O, Guloglu C, Orak M, Sayhan MB, Gokdemir MT, Ustundag M, et al. Trauma scores and neuron-specific enolase, cytokine and C-reactive protein levels as predictors of mortality in patients with blunt head trauma. J Int Med Res. 5:1708–1720. 2010.
Article29. Sripad P, Rosenberg J, Boers F, Filss CP, Galldiks N, Langen KJ, et al. Effect of zolpidem in the aftermath of traumatic brain injury: an MEG study. Case Rep Neurol Med. 2020:8597062. 2020.
Article30. Stahel PF, Shohami E, Younis FM, Kariya K, Otto VI, Lenzlinger PM, et al. Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J Cereb Blood Flow Metab. 20:369–380. 2000.
Article31. Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 34:497–500. 1988.32. Tiret L, Hausherr E, Thicoipe M, Garros B, Maurette P, Castel JP, et al. The epidemiology of head trauma in Aquitaine (France), 1986: a community-based study of hospital admissions and deaths. Int J Epidemiol. 19:133–140. 1990.
Article33. Toplu Y, Sapmaz E, Parlakpinar H, Kelles M, Kalcioglu MT, Tanbek K, et al. The effect of dexpanthenol on ototoxicity induced by cisplatin. Clin Exp Otorhinolaryngol. 9:14–20. 2016.
Article34. Wan J, Ren H, Wang J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc Neurol. 4:93–95. 2019.
Article35. Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 14:128–142. 2013.
Article36. Yon Y, Hernández-García L, Di Giacomo G, Rakovac I, Passmore J, Mikkelsen B. Reducing violence and injury in the WHO European region. Lancet Public Health. 5:e422. 2020.37. Zakaria MM, Hajipour B, Khodadadi A, Afshari F. Ameliorating effects of dexpanthenol in cerebral ischaemia reperfusion induced injury in rat brain. J Pak Med Assoc. 61:889–892. 2011.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Dexpanthenol in Postoperative Patients
- Laser Doppler Estimated Cortical Hemodynamic Changes of Modified Fluid-Percussion Injury Model for Head Trauma Including Hyperosmolar Agent Infusion
- Spinal Cord Injury and Related Clinical Trials
- An Investigation on the Subjective Sequelae of Head Injury
- The Neuroprotective Effect of Intravitreally Injected CNTF on Rat Retinal Ganglion Cell in Optic Nerve Crush Injury Model