Int J Stem Cells.
2024 Aug;17(3):298-308. 10.15283/ijsc23093.
Cytoplasmatic Localization of Six1 in Male Testis and Spermatogonial Stem Cells
- Affiliations
-
- 1Reproductive Medical Center, Affiliated Foshan Maternity & Child Healthcare Hospital, Southern Medical University (Foshan Women and Children Hospital), Foshan, China
- 2State Key Laboratory of Organ Failure Research, Department of Developmental Biology, School of Basic Medical Sciences, Southern Medical University, Guangzhou, China
- 3Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 4Reproductive Medicine Center, Jiangmen Central Hospital, Affiliated Jiangmen Hospital of Sun Yat-sen University, Jiangmen, China
- KMID: 2558607
- DOI: http://doi.org/10.15283/ijsc23093
Abstract
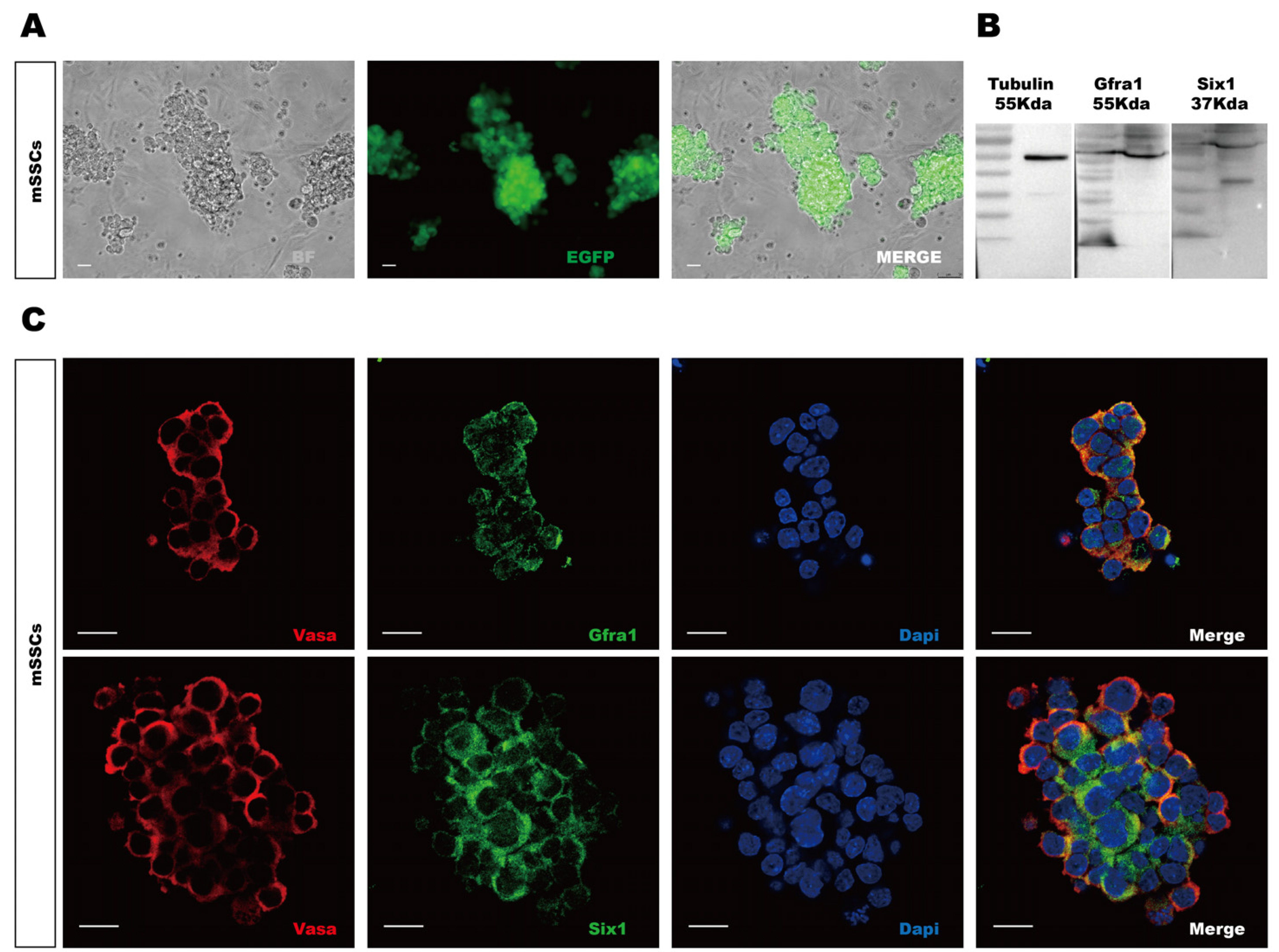
- Sine oculis homeobox 1 (Six1) is an important factor for embryonic development and carcinoma malignancy. However, the localization of Six1 varies due to protein size and cell types in different organs. In this study, we focus on the expression and localization of Six1 in male reproductive organ via bioinformatics analysis and immunofluorescent detection. The potential interacted proteins with Six1 were also predicted by protein-protein interactions (PPIs) and Enrichr analysis. Bioinformatic data from The Cancer Genome Atlas and Genotype-Tissue Expression project databases showed that SIX1 was highly expressed in normal human testis, but low expressed in the testicular germ cell tumor sample. Human Protein Atlas examination verified that SIX1 level was higher in normal than that in cancer samples. The sub-localization of SIX1 in different reproductive tissues varies but specifically in the cytoplasm and membrane in testicular cells. In mouse cells, single cell RNA-sequencing data analysis indicated that Six1 expression level was higher in mouse spermatogonial stem cells (mSSCs) and differentiating spermatogonial than in other somatic cells. Immunofluorescence staining showed the cytoplasmic localization of Six1 in mouse testis and mSSCs. Further PPIs and Enrichr examination showed the potential interaction of Six1 with bone morphogenetic protein 4 (Bmp4) and catenin Beta-1 (CtnnB1) and stem cell signal pathways. Cytoplasmic localization of Six1 in male testis and mSSCs was probably associated with stem cell related proteins Bmp4 and CtnnB1 for stem cell development.
Keyword
Figure
Reference
-
References
1. Kawakami K, Sato S, Ozaki H, Ikeda K. 2000; Six family genes--structure and function as transcription factors and their roles in development. Bioessays. 22:616–626. DOI: 10.1002/1521-1878(200007)22:7<616::AID-BIES4>3.0.CO;2-R. PMID: 10878574.2. Kumar JP. 2009; The sine oculis homeobox (SIX) family of transcription factors as regulators of development and disease. Cell Mol Life Sci. 66:565–583. DOI: 10.1007/s00018-008-8335-4. PMID: 18989625. PMCID: PMC2716997.3. Rafiq A, Aashaq S, Jan I, Beigh MA. 2021; SIX1 transcription factor: a review of cellular functions and regulatory dyna-mics. Int J Biol Macromol. 193(Pt B):1151–1164. DOI: 10.1016/j.ijbiomac.2021.10.133. PMID: 34742853.4. Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. 2003; Six1 is required for the early organogenesis of mammalian kidney. Development. 130:3085–3094. DOI: 10.3410/f.1012294.194261. PMID: 12783782. PMCID: PMC3872112.5. Zeng J, Shi R, Cai CX, et al. 2015; Increased expression of Six1 correlates with progression and prognosis of prostate cancer. Cancer Cell Int. 15:63. DOI: 10.1186/s12935-015-0215-z. PMID: 26161040. PMCID: PMC4497425.6. Wu W, Ren Z, Li P, et al. 2015; Six1: a critical transcription factor in tumorigenesis. Int J Cancer. 136:1245–1253. DOI: 10.1002/ijc.28755. PMID: 24488862.7. Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX. 2012; Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell. 22:377–390. DOI: 10.1016/j.devcel.2011.12.006. PMID: 22340499. PMCID: PMC3285434.8. Kong D, Li A, Liu Y, et al. 2019; SIX1 activates STAT3 signaling to promote the proliferation of thyroid carcinoma via EYA1. Front Oncol. 9:1450. DOI: 10.3389/fonc.2019.01450. PMID: 31921695. PMCID: PMC6933607.9. Wu W, Ren Z, Chen C, et al. 2012; Subcellular localization of different regions of porcine Six1 gene and its expression analysis in C2C12 myoblasts. Mol Biol Rep. 39:9995–10002. DOI: 10.1007/s11033-012-1868-5. PMID: 22752728.10. Zhao J, Lu P, Wan C, et al. 2021; Cell-fate transition and determination analysis of mouse male germ cells throughout deve-lopment. Nat Commun. 12:6839. DOI: 10.1038/s41467-021-27172-0. PMID: 34824237. PMCID: PMC8617176.11. Sisakhtnezhad S. 2018; In silico analysis of single-cell RNA sequencing data from 3 and 7 days old mouse spermatogonial stem cells to identify their differentially expressed genes and transcriptional regulators. J Cell Biochem. 119:7556–7569. DOI: 10.1002/jcb.27066. PMID: 29749669.12. Guo J, Sosa E, Chitiashvili T, et al. 2021; Single-cell analysis of the developing human testis reveals somatic niche cell spe-cification and fetal germline stem cell establishment. Cell Stem Cell. 28:764–778.e4. DOI: 10.1016/j.stem.2020.12.004. PMID: 33453151. PMCID: PMC8026516.13. Zhu G, Liu Y, Zhao L, Lin Z, Piao Y. 2021; The significance of SIX1 as a prognostic biomarker for survival outcome in various cancer patients: a systematic review and meta-anal-ysis. Front Oncol. 11:622331. DOI: 10.3389/fonc.2021.622331. PMID: 34745930. PMCID: PMC8567106.14. Ning L, Goossens E, Geens M, Saen DV, Tournaye H. 2012; Sper-matogonial stem cells as a source for regenerative medicine. Middle East Fertil Soc J. 17:1–7.15. Kanatsu-Shinohara M, Ogonuki N, Inoue K, et al. 2003; Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 69:612–616. DOI: 10.1095/biolreprod.103.017012. PMID: 12700182.16. Guan K, Wolf F, Becker A, Engel W, Nayernia K, Hasenfuss G. 2009; Isolation and cultivation of stem cells from adult mouse testes. Nat Protoc. 4:143–154. DOI: 10.1038/nprot.2008.242. PMID: 19180086.17. Thul PJ, Lindskog C. 2018; The human protein atlas: a spatial map of the human proteome. Protein Sci. 27:233–244. DOI: 10.1002/pro.3307. PMID: 28940711. PMCID: PMC5734309.18. Kanatsu-Shinohara M, Miki H, Inoue K, et al. 2005; Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 72:985–991. DOI: 10.1095/biolreprod.104.036400. PMID: 15601913.19. Wang Y, Ding Y, Li J. 2017; CRISPR-Cas9-mediated gene editing in mouse spermatogonial stem cells. Methods Mol Biol. 1622:293–305. DOI: 10.1007/978-1-4939-7108-4_20. PMID: 28674816.20. Netcharoensirisuk P, Abrahamian C, Tang R, et al. 2021; Flavonoids increase melanin production and reduce proliferation, migration and invasion of melanoma cells by blocking endolysosomal/melanosomal TPC2. Sci Rep. 11:8515. DOI: 10.1038/s41598-021-88196-6. PMID: 33875769. PMCID: PMC8055690.21. Li CY, Cai JH, Tsai JJP, Wang CCN. 2020; Identification of hub genes associated with development of head and neck squamous cell carcinoma by integrated bioinformatics analysis. Front Oncol. 10:681. DOI: 10.3389/fonc.2020.00681. PMID: 32528874. PMCID: PMC7258718.22. Kim JY, Jung HJ, Yoon MJ. 2015; VASA (DDX4) is a putative marker for spermatogonia, spermatocytes and round spermatids in stallions. Reprod Domest Anim. 50:1032–1038. DOI: 10.1111/rda.12632. PMID: 26482643.23. Toyooka Y, Tsunekawa N, Takahashi Y, Matsui Y, Satoh M, Noce T. 2000; Expression and intracellular localization of mouse Vasa-homologue protein during germ cell development. Mech Dev. 93:139–149. DOI: 10.1016/s0925-4773(00)00283-5. PMID: 10781947.24. Ghorbani R, Emamzadeh A, Khazaie Y, et al. 2013; Constructing a mouse Oct4 promoter/EGFP vector, as a whole-cellular reporter to monitor the pluripotent state of cells. Avicenna J Med Biotechnol. 5:2–9. PMID: 23626871. PMCID: PMC3572702.25. Seo HC, Curtiss J, Mlodzik M, Fjose A. 1999; Six class homeobox genes in drosophila belong to three distinct families and are involved in head development. Mech Dev. 83:127–139. DOI: 10.1016/s0925-4773(99)00045-3. PMID: 10381573.26. Coletta RD, Christensen K, Reichenberger KJ, et al. 2004; The Six1 homeoprotein stimulates tumorigenesis by reactiva-tion of cyclin A1. Proc Natl Acad Sci U S A. 101:6478–6483. DOI: 10.1073/pnas.0401139101. PMID: 15123840. PMCID: PMC404070.27. Liu D, Li L, Zhang XX, et al. 2014; SIX1 promotes tumor lymphangiogenesis by coordinating TGFβ signals that increase expression of VEGF-C. Cancer Res. 74:5597–5607. Erratum in: Cancer Res 2019;79:1715. DOI: 10.1158/0008-5472.can-13-3598. PMID: 25142796.28. Wang CA, Jedlicka P, Patrick AN, et al. 2012; SIX1 induces lymphangiogenesis and metastasis via upregulation of VEGF-C in mouse models of breast cancer. J Clin Invest. 122:1895–1906. DOI: 10.1172/jci59858. PMID: 22466647. PMCID: PMC3336979.29. Kong J, Zhou X, Liu S, et al. 2014; Overexpression of sineoculis homeobox homolog 1 predicts poor prognosis of hepato-cellular carcinoma. Int J Clin Exp Pathol. 7:3018–3027. PMID: 25031720. PMCID: PMC4097294.30. Patrick AN, Cabrera JH, Smith AL, Chen XS, Ford HL, Zhao R. 2013; Structure-function analyses of the human SIX1-EYA2 complex reveal insights into metastasis and BOR syndrome. Nat Struct Mol Biol. 20:447–453. DOI: 10.1038/nsmb.2505. PMID: 23435380. PMCID: PMC3618615.31. Fujimoto Y, Tanaka SS, Yamaguchi YL, et al. 2013; Homeopro-teins Six1 and Six4 regulate male sex determination and mouse gonadal development. Dev Cell. 26:416–430. DOI: 10.1016/j.devcel.2013.06.018. PMID: 23987514.32. Hansen GM, Lawler ME, Williams WB, Troulis MJ, Kaban LB. 2012; BMP4 localization and PCNA expression during distraction osteogenesis of the porcine mandible. Int J Oral Maxillofac Surg. 41:867–873. DOI: 10.1016/j.ijom.2011.12.032. PMID: 22285012.33. Haid B, Pechriggl E, Nägele F, et al. 2020; FGF8, FGF10 and FGF receptor 2 in foreskin of children with hypospadias: an analysis of immunohistochemical expression patterns and gene transcription. J Pediatr Urol. 16:41.e1–41.e10. DOI: 10.1016/j.jpurol.2019.10.007. PMID: 31718875.34. Luo S, Liu Z, Bian Q, Wang X. 2023; Ectomesenchymal Six1 controls mandibular skeleton formation. Front Genet. 14:1082911. DOI: 10.3389/fgene.2023.1082911. PMID: 36845386. PMCID: PMC9946248.35. Terakawa J, Serna VA, Nair DM, et al. 2020; SIX1 cooperates with RUNX1 and SMAD4 in cell fate commitment of Müllerian duct epithelium. Cell Death Differ. 27:3307–3320. DOI: 10.1038/s41418-020-0579-z. PMID: 32572167. PMCID: PMC7852590.36. Kurek D, Neagu A, Tastemel M, et al. 2015; Endogenous WNT signals mediate BMP-induced and spontaneous differentia-tion of epiblast stem cells and human embryonic stem cells. Stem Cell Reports. 4:114–128. DOI: 10.1016/j.stemcr.2014.11.007. PMID: 25544567. PMCID: PMC4297870.37. Takase HM, Nusse R. 2016; Paracrine Wnt/β-catenin signaling mediates proliferation of undifferentiated spermatogonia in the adult mouse testis. Proc Natl Acad Sci U S A. 113:E1489–E1497. DOI: 10.1073/pnas.1601461113. PMID: 26929341. PMCID: PMC4801309.38. McCoy EL, Iwanaga R, Jedlicka P, et al. 2009; Six1 expands the mouse mammary epithelial stem/progenitor cell pool and induces mammary tumors that undergo epithelial-mesenchymal transition. J Clin Invest. 119:2663–2677. DOI: 10.1172/JCI37691. PMID: 19726883. PMCID: PMC2735909.39. Wang Z, Yang Y, Hu S, et al. 2021; Short-form RON (sf-RON) enhances glucose metabolism to promote cell proliferation via activating β-catenin/SIX1 signaling pathway in gastric cancer. Cell Biol Toxicol. 37:35–49. DOI: 10.1007/s10565-020-09525-5. PMID: 32399910. PMCID: PMC7851020.40. Song W, Ma J, Lei B, et al. 2019; Sine oculis homeobox 1 promotes proliferation and migration of human colorectal cancer cells through activation of Wnt/β-catenin signaling. Cancer Sci. 110:608–616. DOI: 10.1111/cas.13905. PMID: 30548112. PMCID: PMC6361609.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Class I Histone Deacetylase Enhances Self-Reprogramming of Spermatogonial Stem Cells into Pluripotent Stem Cells
- Establishment of a surgically induced cryptorchidism canine recipient model for spermatogonial stem cell transplantation
- Dimethyloxaloylglycine promotes spermatogenesis activity of spermatogonial stem cells in Bama minipigs
- Etv5, a transcription factor with versatile functions in male reproduction
- Enrichment and In Vitro Culture of Spermatogonial Stem Cells from Pre-Pubertal Monkey Testes