Nutr Res Pract.
2024 Jun;18(3):436-445. 10.4162/nrp.2024.18.3.436.
A combination of myokines and genistein suppresses cancer stemness in MCF-7 human breast cancer cells
- Affiliations
-
- 1Graduate Program in System Health Science and Engineering, Ewha Womans University, Seoul 03760, Korea
- 2Department of Nutritional Science and Food Management, Ewha Womans University, Seoul 03760, Korea
- 3Department of Physical Education, Chung-Ang University, Seoul 06974, Korea
- KMID: 2558515
- DOI: http://doi.org/10.4162/nrp.2024.18.3.436
Abstract
- BACKGROUND/OBJECTIVES
Breast cancer is considered a serious health issue worldwide and is influenced by risk factors, including physical inactivity and unhealthy diet. Myokines secreted by muscles during physical activity play a crucial role in cancer development and the immune system. Genistein (Gen), an isoflavone primarily in legumes, induces anti-cancer activity by regulating cancer stem cells (CSCs). Therefore, this study investigated the potential anti-cancer effect of a combination of myokine and Gen on the human breast cancer MCF-7 cells.
MATERIALS/METHODS
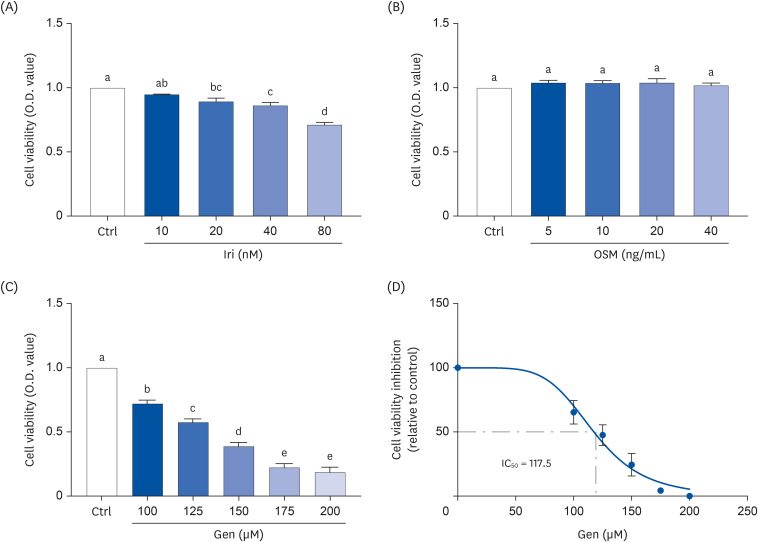
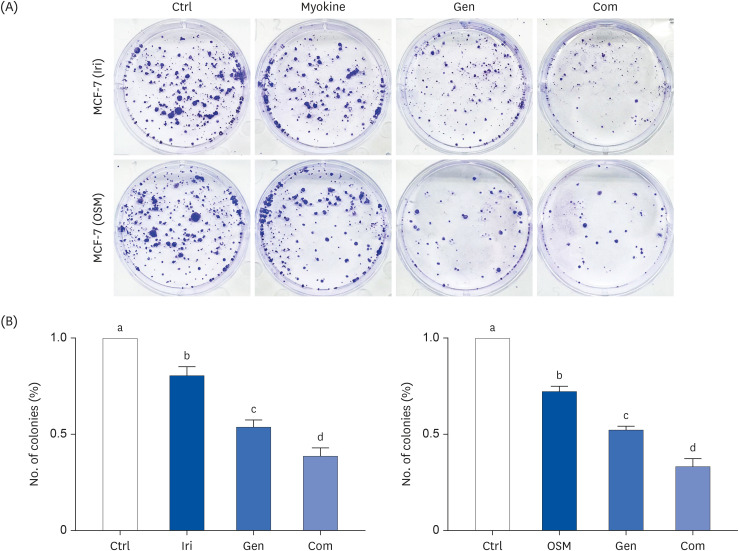
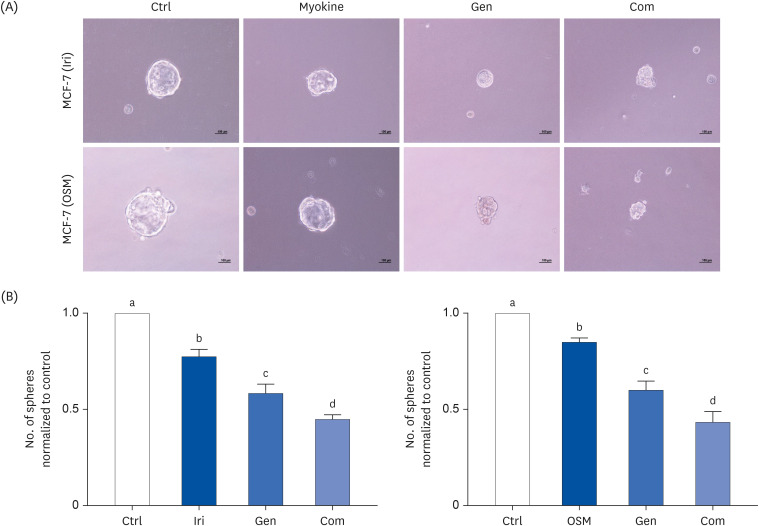
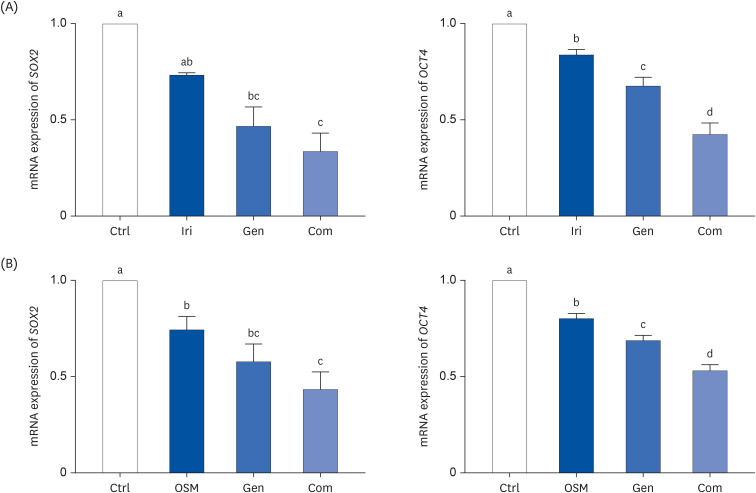
MCF-7, a human breast cancer cell line, was used for in vitro study. The cell viability of MCF-7 cells was evaluated in response to treatment with myokines, irisin (Iri), oncostatin M (OSM), and Gen using the MTT assay. Clonogenic and sphere formation assays were used to evaluate the self-renewal capacity of breast CSCs. The mRNA expression levels of stem cell markers were analyzed in MCF-7 breast cancer cells.
RESULTS
Administering Iri or OSM with Gen significantly inhibited the self-renewal capacity of MCF-7 cells. In addition, mRNA expression of breast CSC markers SOX2 and OCT4, which are characteristic of CSCs, was suppressed by both myokine and Gen. However, combining Iri or OSM with Gen was the most effective treatment.
CONCLUSION
These results suggested that combining Iri or OSM with Gen has an additive effect on breast CSCs by regulating self-renewal capacity and expression of CSCs markers. Therefore, the combination of myokines and Gen may have the therapeutic potential for treating breast cancer and improving the quality of life of cancer patients.
Keyword
Figure
Reference
-
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023; 73:17–48. PMID: 36633525.2. Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020; 8:e1027–e1037. PMID: 32710860.3. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010; 19:1893–1907. PMID: 20647400.4. Gopinath A, Cheema AH, Chaludiya K, Khalid M, Nwosu M, Agyeman WY, Bisht A, Venugopal S. The impact of dietary fat on breast cancer incidence and survival: a systematic review. Cureus. 2022; 14:e30003. PMID: 36381753.5. Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018; 25:20. PMID: 29506506.6. Aponte PM, Caicedo A. Stemness in cancer: stem cells, cancer stem cells, and their microenvironment. Stem Cells Int. 2017; 2017:5619472. PMID: 28473858.7. Chang JC. Cancer stem cells: role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine (Baltimore). 2016; 95:S20–S25. PMID: 27611935.8. Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002; 132:3456S–3464S. PMID: 12421870.9. Jiang Y, Wang W. Potential mechanisms of cancer prevention by weight control. Biophys Rev Lett. 2008; 3:421–437.10. Díaz BB, González DA, Gannar F, Pérez MCR, de León AC. Myokines, physical activity, insulin resistance and autoimmune diseases. Immunol Lett. 2018; 203:1–5. PMID: 30194964.11. Hojman P. Exercise protects from cancer through regulation of immune function and inflammation. Biochem Soc Trans. 2017; 45:905–911. PMID: 28673937.12. Hojman P, Dethlefsen C, Brandt C, Hansen J, Pedersen L, Pedersen BK. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am J Physiol Endocrinol Metab. 2011; 301:E504–E510. PMID: 21653222.13. Cebulski K, Nowińska K, Jablońska K, Romanowicz H, Smolarz B, Dzięgiel P, Podhorska-Okołów M. Expression of irisin/FNDC5 in breast cancer. Int J Mol Sci. 2022; 23:23.14. Masjedi A, Hajizadeh F, Beigi Dargani F, Beyzai B, Aksoun M, Hojjat-Farsangi M, Zekiy A, Jadidi-Niaragh F, Oncostatin M. Oncostatin M: a mysterious cytokine in cancers. Int Immunopharmacol. 2021; 90:107158. PMID: 33187910.15. Hutt JA, DeWille JW. Oncostatin M induces growth arrest of mammary epithelium via a CCAAT/enhancer-binding protein delta-dependent pathway. Mol Cancer Ther. 2002; 1:601–610. PMID: 12479220.16. Holzer RG, Ryan RE, Tommack M, Schlekeway E, Jorcyk CL. Oncostatin M stimulates the detachment of a reservoir of invasive mammary carcinoma cells: role of cyclooxygenase-2. Clin Exp Metastasis. 2004; 21:167–176. PMID: 15168734.17. Haider T, Pandey V, Banjare N, Gupta PN, Soni V. Drug resistance in cancer: mechanisms and tackling strategies. Pharmacol Rep. 2020; 72:1125–1151. PMID: 32700248.18. Mukund V. Genistein: its role in breast cancer growth and metastasis. Curr Drug Metab. 2020; 21:6–10. PMID: 31987018.19. Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008; 269:226–242. PMID: 18492603.20. Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA, Ahmad B, Atif M, Mubarak MS, Sytar O, et al. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid Med Cell Longev. 2021; 2021:3268136. PMID: 34336089.21. Goh YX, Jalil J, Lam KW, Husain K, Premakumar CM. Genistein: a review on its anti-inflammatory properties. Front Pharmacol. 2022; 13:820969. PMID: 35140617.22. Sutrisno S, Aprina H, Simanungkalit HM, Andriyani A, Barlianto W, Sujuti H, Santoso S, Dwijayasa PM, Wahyuni ES, Mustofa E. Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. J Tradit Complement Med. 2017; 8:278–281. PMID: 29736382.23. Wang B, Xu H, Hu X, Ma W, Zhang J, Li Y, Yu M, Zhang Y, Li X, Ye X. Synergetic inhibition of daidzein and regular exercise on breast cancer in bearing-4T1 mice by regulating NK cells and apoptosis pathway. Life Sci. 2020; 245:117387. PMID: 32007575.24. Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro . Nat Protoc. 2006; 1:2315–2319. PMID: 17406473.25. Song S, Seo D. Cancer stem cells: biological features and targeted therapeutics. Hanyang Med Rev. 2015; 35:250.26. Fan P, Fan S, Wang H, Mao J, Shi Y, Ibrahim MM, Ma W, Yu X, Hou Z, Wang B, et al. Genistein decreases the breast cancer stem-like cell population through Hedgehog pathway. Stem Cell Res Ther. 2013; 4:146. PMID: 24331293.27. Shao L, Li H, Chen J, Song H, Zhang Y, Wu F, Wang W, Zhang W, Wang F, Li H, et al. Irisin suppresses the migration, proliferation, and invasion of lung cancer cells via inhibition of epithelial-to-mesenchymal transition. Biochem Biophys Res Commun. 2017; 485:598–605. PMID: 27986567.28. Yamashita T, Honda M, Nio K, Nakamoto Y, Yamashita T, Takamura H, Tani T, Zen Y, Kaneko S. Oncostatin m renders epithelial cell adhesion molecule-positive liver cancer stem cells sensitive to 5-fluorouracil by inducing hepatocytic differentiation. Cancer Res. 2010; 70:4687–4697. PMID: 20484035.29. Li C, Ahlborn TE, Kraemer FB, Liu J. Oncostatin M-induced growth inhibition and morphological changes of MDA-MB231 breast cancer cells are abolished by blocking the MEK/ERK signaling pathway. Breast Cancer Res Treat. 2001; 66:111–121. PMID: 11437097.30. Ma W, Zhang Y, Yu M, Wang B, Xu S, Zhang J, Li X, Ye X. In-vitro and in-vivo anti-breast cancer activity of synergistic effect of berberine and exercise through promoting the apoptosis and immunomodulatory effects. Int Immunopharmacol. 2020; 87:106787. PMID: 32707493.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Cyclooxygenase-2 (Cox-2) Expression by Genistein in Breast Cancer Cell-line
- Alfa - difluoromethylornithine Reduced Protein Phosphorylation in MCF-7 Human Breast Cancer Cells
- Apoptotic Effects of 6-Gingerol in Human Breast Cancer Cells
- Comparative Studies on the Polyarnine Involvement in MCF - 7 and MDA - MB - 231 Breast Cancer Cell Proliferation
- Different Expression of p27(kip1) and p57(kip2) by Genistein Treatment in MDA-MB-231 Human Breast Cancer Cells