Nutr Res Pract.
2024 Jun;18(3):309-324. 10.4162/nrp.2024.18.3.309.
Anti-osteoporotic effects of Boswellia serrata gum resin extract in vitro and in vivo
- Affiliations
-
- 1Department of Food Science & Nutrition, Dongseo University, Busan 47011, Korea
- 2Industry coupled Cooperation Center for Bio Healthcare Materials, Hallym University, Chuncheon 24252, Korea
- 3Health Functional Food Material Development Team, Bio Lab., Frombio Co., Ltd., Yongin 17108, Korea
- KMID: 2558506
- DOI: http://doi.org/10.4162/nrp.2024.18.3.309
Abstract
- BACKGROUND/OBJECTIVES
This study evaluated the beneficial effects of an ethanol extract of Boswellia serrata gum resin (FJH-UBS) in osteoporosis.
MATERIALS/METHODS
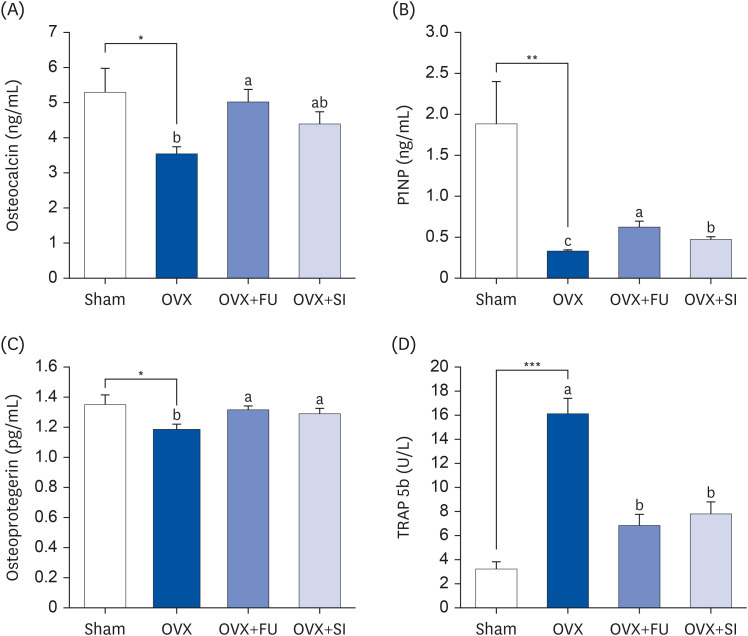
MC3T3-E1 osteoblastic cells and RAW 264.7 osteoclastic cells were treated with FJH-UBS. The alkaline phosphatase (ALP) activity, mineralization, collagen synthesis, osteocalcin content, and Runt-related transcription factor 2 (RUNX2) and Osterix expression were measured in MC3T3-E1 cells. The actin ring structures, tartrate-resistant acid phosphatase (TRAP) activity, and the nuclear factor of activator T-cells, cytoplasm 1 (NFATc1) expression were evaluated in RAW 264.7 cells. Ovariectomized ICR mice were orally administered FJH-UBS for eight weeks. The bone mineral density (BMD) and the serum levels of osteocalcin, procollagen 1 N-terminal propeptide (P1NP), osteoprotegerin, and TRAP 5b were analyzed.
RESULTS
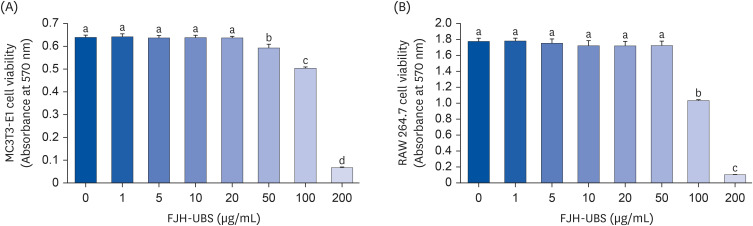
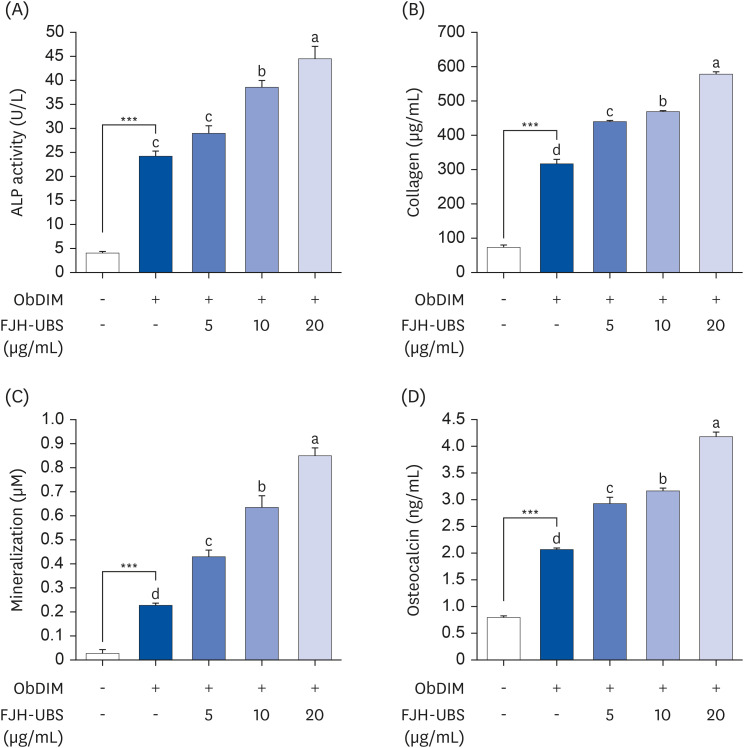
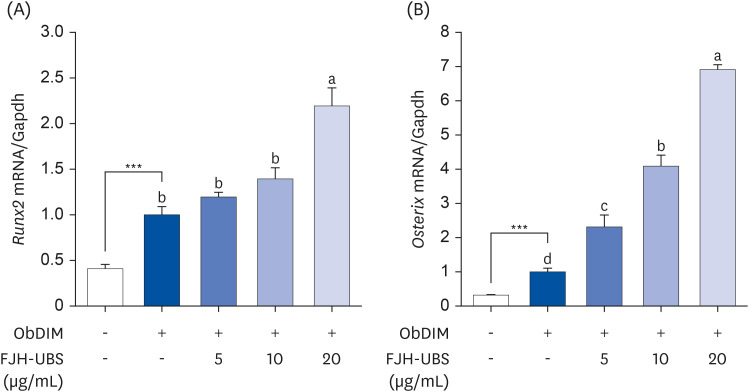
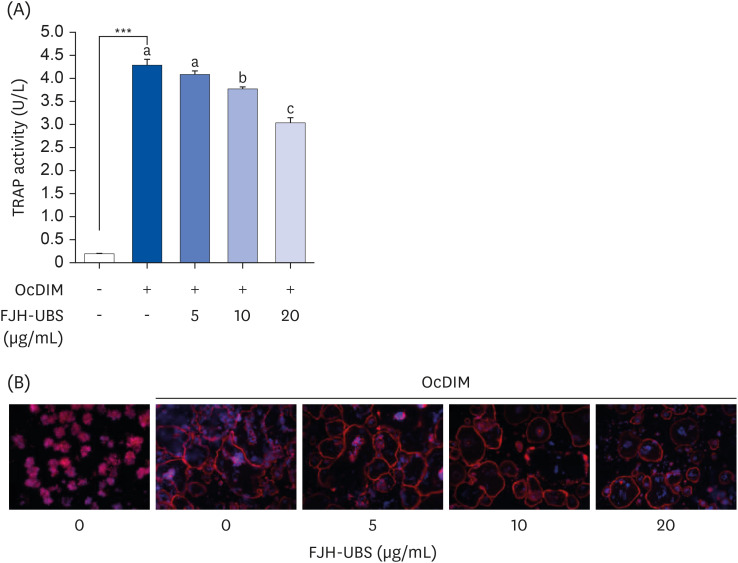
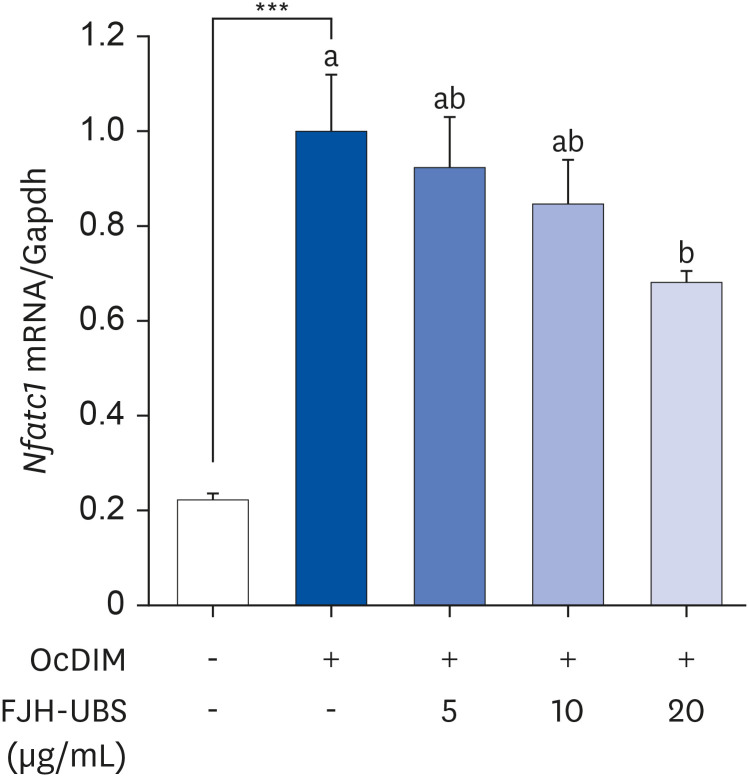
FJH-UBS increased the ALP activity, collagen, osteocalcin, mineralization, and RUNX2 and osterix expression in MC3T3-E1 osteoblastic cells, whereas it decreased the TRAP activity, actin ring structures, and NFATc1 expression in RAW 264.7 osteoclastic cells. In ovariectomy-induced osteoporosis mice, FJH-UBS positively restored all of the changes in the bone metabolism biomarkers (BMD, osteocalcin, P1NP, osteoprotegerin, and TRAP 5b) caused by the ovariectomy.
CONCLUSION
FJH-UBS has anti-osteoporotic activity by promoting osteoblast activity and inhibiting osteoclast activity in vitro and in vivo, suggesting that FJH-UBS is a potential functional food ingredient for osteoporosis.
Keyword
Figure
Reference
-
1. Hwang YH, Kim KJ, Kim SJ, Mun SK, Hong SG, Son YJ, Yee ST. Suppression effect of astaxanthin on osteoclast formation in vitro and bone loss in vivo . Int J Mol Sci. 2018; 19:912. PMID: 29562730.2. Langdahl BL. Overview of treatment approaches to osteoporosis. Br J Pharmacol. 2021; 178:1891–1906. PMID: 32060897.3. Li SS, He SH, Xie PY, Li W, Zhang XX, Li TF, Li DF. Recent progresses in the treatment of osteoporosis. Front Pharmacol. 2021; 12:717065. PMID: 34366868.4. He JB, Chen MH, Lin DK. New insights into the tonifying kidney-yin herbs and formulas for the treatment of osteoporosis. Arch Osteoporos. 2017; 12:14. PMID: 28127706.5. Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, Kavanagh KL, Triffitt JT, Lundy MW, Phipps RJ, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007; 1117:209–257. PMID: 18056045.6. O’Ryan FS, Lo JC. Bisphosphonate-related osteonecrosis of the jaw in patients with oral bisphosphonate exposure: clinical course and outcomes. J Oral Maxillofac Surg. 2012; 70:1844–1853. PMID: 22595135.7. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002; 288:321–333. PMID: 12117397.8. Lee J, Jeong I, Kim GW, Son T, Kim Y, Jun W, Park J, Kim OK. Standardized ethanolic extracts of Boswellia serrata ameliorate symptoms of osteoarthritis by direct effects on chondrocytes. J Food Nutr Res. 2021; 9:614–625.9. Majeed M, Majeed S, Narayanan NK, Nagabhushanam K. A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phytother Res. 2019; 33:1457–1468. PMID: 30838706.10. Mannino G, Occhipinti A, Maffei ME. Quantitative determination of 3-O-acetyl-11-keto-β boswellic Acid (AKBA) and other boswellic acids in Boswellia sacra Flueck (syn. B. carteri Birdw) and Boswellia serrata Roxb. Molecules. 2016; 21:1329. PMID: 27782055.11. Roy NK, Parama D, Banik K, Bordoloi D, Devi AK, Thakur KK, Padmavathi G, Shakibaei M, Fan L, Sethi G, et al. An update on pharmacological potential of boswellic acids against chronic diseases. Int J Mol Sci. 2019; 20:4101. PMID: 31443458.12. Zhang J, Zhao J, Sun Y, Liang Y, Zhao J, Zou H, Zhang T, Ren L. GR-mediated anti-inflammation of α-boswellic acid: insights from in vitro and in silico studies. Food Chem Toxicol. 2021; 155:112379. PMID: 34197882.13. Zimmermann-Klemd AM, Reinhardt JK, Nilsu T, Morath A, Falanga CM, Schamel WW, Huber R, Hamburger M, Gründemann C. Boswellia carteri extract and 3-O-acetyl-alpha-boswellic acid suppress T cell function. Fitoterapia. 2020; 146:104694. PMID: 32712132.14. Bharat KT, Manhas NS, Gutcho J, Lin J, Bhattacharyya S, Kounang R. Ingredients of a natural oral nutritional supplement and their role in the treatment of osteoarthritis. Clin Med Insights Arthritis Musculoskelet Disord. 2022; 15:11795441211063365. PMID: 35360183.15. Mariano A, Bigioni I, Misiti F, Fattorini L, Scotto D’Abusco A, Rodio A. The nutraceuticals as modern key to achieve erythrocyte oxidative stress fighting in osteoarthritis. Curr Issues Mol Biol. 2022; 44:3481–3495. PMID: 36005136.16. Al-Dhubiab BE, Patel SS, Morsy MA, Duvva H, Nair AB, Deb PK, Shah J. The beneficial effect of boswellic acid on bone metabolism and possible mechanisms of action in experimental osteoporosis. Nutrients. 2020; 12:3186. PMID: 33081068.17. Takada Y, Ichikawa H, Badmaev V, Aggarwal BB. Acetyl-11-keto-beta-boswellic acid potentiates apoptosis, inhibits invasion, and abolishes osteoclastogenesis by suppressing NF-kappa B and NF-kappa B-regulated gene expression. J Immunol. 2006; 176:3127–3140. PMID: 16493072.18. Xiong L, Liu Y, Zhu F, Lin J, Wen D, Wang Z, Bai J, Ge G, Xu C, Gu Y, et al. Acetyl-11-keto-β-boswellic acid attenuates titanium particle-induced osteogenic inhibition via activation of the GSK-3β/β-catenin signaling pathway. Theranostics. 2019; 9:7140–7155. PMID: 31695758.19. Jung JI, Lee HS, Kim R, Kim EJ. Anti-osteoarthritis effect of enriched Boswellia serrata gum resin extract in SW1353 chondrocytes. J Korean Soc Food Sci Nutr. 2023; 52:460–472.20. Jung JI, Kim R, Kim EJ. Anti-osteoarthritis effect of Boswellia serrata gum resin extract in monosodium iodoacetate-induced osteoarthritic Sprague-Dawley rats. J Nutr Health. 2023; 56:231–246.21. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986; 89:271–277. PMID: 3486233.22. Lim SM, Jung JI, Kim NY, Bae JS, Lee HS, Kim EJ. Cyanidine-3-O-galactoside enriched Aronia melanocarpa extract inhibits adipogenesis and lipogenesis via down-regulation of adipogenic transcription factors and their target genes in 3T3-L1 cells. Food Nutr Sci. 2019; 10:128–147.23. Kim EJ, Jung JI, Jeon YE, Lee HS. Aqueous extract of Petasites japonicus leaves promotes osteoblast differentiation via up-regulation of Runx2 and Osterix in MC3T3-E1 cells. Nutr Res Pract. 2021; 15:579–590. PMID: 34603606.24. Souza VR, Mendes E, Casaro M, Antiorio AT, Oliveira FA, Ferreira CM. Description of ovariectomy protocol in mice. Methods Mol Biol. 2019; 1916:303–309. PMID: 30535707.25. Soysa NS, Alles N. Osteoclast function and bone-resorbing activity: an overview. Biochem Biophys Res Commun. 2016; 476:115–120. PMID: 27157135.26. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011; 6:121–145. PMID: 20936937.27. Fontalis A, Kenanidis E, Prousali E, Potoupnis M, Tsiridis E. Safety and efficacy of denosumab in osteoporotic patients previously treated with other medications: a systematic review and meta-analysis. Expert Opin Drug Saf. 2018; 17:413–428. PMID: 29350565.28. Singh P, Chacko KM, Aggarwal ML, Bhat B, Khandal RK, Sultana S, Kuruvilla BT. A-90 day gavage safety assessment of Boswellia serrata in rats. Toxicol Int. 2012; 19:273–278. PMID: 23293466.29. Dodda S, Madireddy RK, Alluri VK, Golakoti T, Sengupta K. Safety assessment of a novel water-soluble extract of Boswellia serrata gum resin: acute toxicity, 90-day sub-chronic toxicity, Ames’ bacterial reverse mutation, and in vivo micronucleus assays. Toxicol Mech Methods. 2022; 32:362–372. PMID: 34886755.30. Sharma T, Jana S. Investigation of molecular properties that influence the permeability and oral bioavailability of major β-boswellic acids. Eur J Drug Metab Pharmacokinet. 2020; 45:243–255. PMID: 31786725.31. Kim WJ, Shin HL, Kim BS, Kim HJ, Ryoo HM. RUNX2-modifying enzymes: therapeutic targets for bone diseases. Exp Mol Med. 2020; 52:1178–1184. PMID: 32788656.32. Liu Q, Li M, Wang S, Xiao Z, Xiong Y, Wang G. Recent advances of osterix transcription factor in osteoblast differentiation and bone formation. Front Cell Dev Biol. 2020; 8:601224. PMID: 33384998.33. Barbuto R, Mitchell J. Regulation of the osterix (Osx, Sp7) promoter by osterix and its inhibition by parathyroid hormone. J Mol Endocrinol. 2013; 51:99–108. PMID: 23682129.34. Hayman AR. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity. 2008; 41:218–223. PMID: 18365835.35. Zhao Q, Wang X, Liu Y, He A, Jia R. NFATc1: functions in osteoclasts. Int J Biochem Cell Biol. 2010; 42:576–579. PMID: 20035895.36. Zheng H, Qi S, Chen C. Salidroside improves bone histomorphology and prevents bone loss in ovariectomized diabetic rats by upregulating the OPG/RANKL ratio. Molecules. 2018; 23:2398. PMID: 30235836.37. Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med. 2011; 171:1363–1369. PMID: 21824950.38. Qi S, Zheng H. Combined effects of phytoestrogen genistein and silicon on ovariectomy-induced bone loss in rat. Biol Trace Elem Res. 2017; 177:281–287. PMID: 27785742.39. Kim SM, Lee HS, Jung JI, Lim SM, Lim JH, Ha WH, Jeon CL, Lee JY, Kim EJ. Effect of isoflavone-enriched whole soy milk powder supplementation on bone metabolism in ovariectomized mice. Nutr Res Pract. 2018; 12:275–282. PMID: 30090164.40. Yousefzadeh N, Kashfi K, Jeddi S, Ghasemi A. Ovariectomized rat model of osteoporosis: a practical guide. EXCLI J. 2020; 19:89–107. PMID: 32038119.41. Fu SW, Zeng GF, Zong SH, Zhang ZY, Zou B, Fang Y, Lu L, Xiao DQ. Systematic review and meta-analysis of the bone protective effect of phytoestrogens on osteoporosis in ovariectomized rats. Nutr Res. 2014; 34:467–477. PMID: 25026913.42. Bonjour JP, Kohrt W, Levasseur R, Warren M, Whiting S, Kraenzlin M. Biochemical markers for assessment of calcium economy and bone metabolism: application in clinical trials from pharmaceutical agents to nutritional products. Nutr Res Rev. 2014; 27:252–267. PMID: 25394580.43. Kuo TR, Chen CH. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark Res. 2017; 5:18. PMID: 28529755.44. Gillett MJ, Vasikaran SD, Inderjeeth CA. The role of PINP in diagnosis and management of metabolic bone disease. Clin Biochem Rev. 2021; 42:3–10. PMID: 34305208.45. Chen FP, Fu TS, Lin YC, Sung CM, Huang MH, Lin YJ. Association between P1NP and bone strength in postmenopausal women treated with teriparatide. Taiwan J Obstet Gynecol. 2022; 61:91–95. PMID: 35181054.46. Chen CY, Rao SS, Tan YJ, Luo MJ, Hu XK, Yin H, Huang J, Hu Y, Luo ZW, Liu ZZ, et al. Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res. 2019; 7:18. PMID: 31263627.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-osteoarthritis effect of Boswellia serrata gum resin extract in monosodium iodoacetate-induced osteoarthritic Sprague-Dawley rats
- Olibanum Extract Inhibits Vascular Smooth Muscle Cell Migration and Proliferation in Response to Platelet-Derived Growth Factor
- Anti-toxoplasmosis effect of Dictamnus dasycarpus extract against Toxoplasma Gondii
- In vitro and in vivo antibacterial activity of Meliae fructus extract against Helicobacter pylori
- Anti-Toxoplasmosis Effect of Meliae fructus Ethanol Extract