Nutr Res Pract.
2024 Aug;18(4):498-510. 10.4162/nrp.2024.18.4.498.
Opuntia humifusa stems rich in quercetin and isorhamnetin alleviate insulin resistance in high-fat diet-fed rats
- Affiliations
-
- 1Department of Practical Science Education, Gyeongin National University of Education, Incheon 21044, Korea
- 2Department of Food and Nutrition, Gyeongsang National University, Jinju 52828, Korea
- 3Functional Food Division, National Institute of Agricultural Sciences, Rural Development Administration, Wanju 55365, Korea
- 4Department of Food and Nutrition, Institute of Agriculture and Life Science, Gyeongsang National University, Jinju 52828, Korea
- KMID: 2558488
- DOI: http://doi.org/10.4162/nrp.2024.18.4.498
Abstract
- BACKGROUND/OBJECTIVES
Obesity, characterized by abnormal fat accumulation and metabolic disturbances, presents a significant health challenge. Opuntia humifusa Raf., commonly known as Korean Cheonnyuncho, is rich in various beneficial compounds and has demonstrated antioxidant and anti-inflammatory effects. However, its potential impact on glucose and lipid metabolism, particularly in obese rats, remains unexplored. We aimed to investigate whether O. humifusa stems and fruits could beneficially alter glucose metabolism and lipid profiles in a rat model of high-fat diet (HFD)-induced obesity.
MATERIALS/METHODS
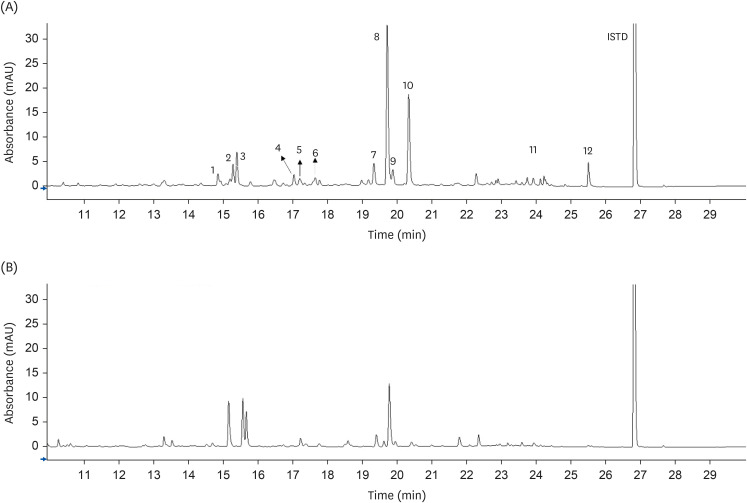
Thirty-two rats were allocated into 4 groups: normal diet (NF), HFD control (HF), HFD treated with 2% O. humifusa stems (HF-OS), and HFD treated with 2% O. humifusa fruits (HF-OF). Experimental diets were administered for 6 weeks. At the end of the treatment, liver and fat tissues were isolated, and serum was collected for biochemical analysis. The major flavonoid from O. humifusa stems and fruits was identified and quantified.
RESULTS
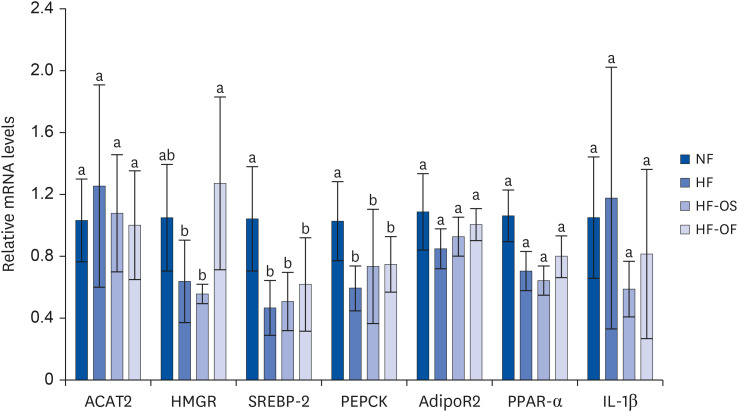
After 6 weeks of treatment, the serum fasting glucose concentration in the HF-OS group was significantly lower than that in the HF group. Serum fasting insulin concentrations in both HF-OS and HF-OF groups tended to be lower than those in the HF group, indicating a significant improvement in insulin sensitivity in the HF-OS group. Additionally, the HF-OS group exhibited a tendency towards the restoration of adiponectin levels to that of the NF group.
CONCLUSION
The 2% O. humifusa stems contain abundant quercetin and isorhamnetin, which alter fasting blood glucose levels in rats fed a HFD, leading to a favorable improvement in insulin resistance.
Keyword
Figure
Reference
-
1. Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022; 133:155217. PMID: 35584732.
Article2. Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012; 126:126–132. PMID: 22753534.
Article3. Woolcott OO, Seuring T. Prevalence trends in obesity defined by the relative fat mass (RFM) index among adults in the United States: 1999–2018. Metabolism. 2022; 128:155027.
Article4. Kivimäki M, Strandberg T, Pentti J, Nyberg ST, Frank P, Jokela M, Ervasti J, Suominen SB, Vahtera J, Sipilä PN, et al. Body-mass index and risk of obesity-related complex multimorbidity: an observational multicohort study. Lancet Diabetes Endocrinol. 2022; 10:253–263. PMID: 35248171.5. Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018; 34:575–584. PMID: 29459239.
Article6. Emerging Risk Factors Collaboration. Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011; 377:1085–1095. PMID: 21397319.
Article7. Mayor S. Being overweight may raise risk of eight more cancers, review finds. BMJ. 2016; 354:i4650. PMID: 27561683.
Article8. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010; 51:679–689. PMID: 20041406.
Article9. Francque SM, Dirinck E. NAFLD prevalence and severity in overweight and obese populations. Lancet Gastroenterol Hepatol. 2023; 8:2–3. PMID: 36400096.10. Tyrrell J, Mulugeta A, Wood AR, Zhou A, Beaumont RN, Tuke MA, Jones SE, Ruth KS, Yaghootkar H, Sharp S, et al. Using genetics to understand the causal influence of higher BMI on depression. Int J Epidemiol. 2019; 48:834–848. PMID: 30423117.
Article11. Mili N, Paschou SA, Goulis DG, Dimopoulos MA, Lambrinoudaki I, Psaltopoulou T. Obesity, metabolic syndrome, and cancer: pathophysiological and therapeutic associations. Endocrine. 2021; 74:478–497. PMID: 34625915.
Article12. Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia. 2008; 51:1781–1789. PMID: 18726585.
Article13. Larsson SC, Burgess S. Causal role of high body mass index in multiple chronic diseases: a systematic review and meta-analysis of Mendelian randomization studies. BMC Med. 2021; 19:320. PMID: 34906131.
Article14. Kotsis V, Jordan J, Micic D, Finer N, Leitner DR, Toplak H, Tokgozoglu L, Athyros V, Elisaf M, Filippatos TD, et al. Obesity and cardiovascular risk: a call for action from the European Society of Hypertension Working Group of Obesity, Diabetes and the High-risk Patient and European Association for the Study of Obesity: part A: mechanisms of obesity induced hypertension, diabetes and dyslipidemia and practice guidelines for treatment. J Hypertens. 2018; 36:1427–1440. PMID: 29634663.15. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013; 309:71–82. PMID: 23280227.
Article16. Jia H, Lubetkin EI. Trends in quality-adjusted life-years lost contributed by smoking and obesity. Am J Prev Med. 2010; 38:138–144. PMID: 20117569.
Article17. Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev. 2001; 2:219–229. PMID: 12119993.
Article18. Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009; 28:w822–w831. PMID: 19635784.
Article19. Okunogbe A, Nugent R, Spencer G, Ralston J, Wilding J. Economic impacts of overweight and obesity: current and future estimates for eight countries. BMJ Glob Health. 2021; 6:e006351.20. Okunogbe A, Nugent R, Spencer G, Powis J, Ralston J, Wilding J. Economic impacts of overweight and obesity: current and future estimates for 161 countries. BMJ Glob Health. 2022; 7:e009773.
Article21. Anderson EF. The Cactus Family. Portland (OR): Timber Press;2001.22. Goldstein G, Nobel PS. Water relations and low-temperature acclimation for cactus species varying in freezing tolerance. Plant Physiol. 1994; 104:675–681. PMID: 12232118.
Article23. Del Socorro Santos Díaz M, Barba de la Rosa AP, Héliès-Toussaint C, Guéraud F, Nègre-Salvayre A. Opuntia spp.: characterization and benefits in chronic diseases. Oxid Med Cell Longev. 2017; 2017:8634249. PMID: 28491239.24. Yoon JA, Hahm SW, Park JE, Son YS. Total polyphenol and flavonoid of fruit extract of Opuntia humifusa and its inhibitory effect on the growth of MCF-7 human breast cancer cells. J Korean Soc Food Sci Nutr. 2009; 38:1679–1684.25. Yoon JA, Hahm SW, Son YS. Nutrients contents in different parts of pickly pear (Opuntia humifusa) and possible anti-breast cancer effect. J Korean Soc Food Sci Nutr. 2009; 22:485–491.26. Hahm SW, Park J, Son YS. Opuntia humifusa stems lower blood glucose and cholesterol levels in streptozotocin-induced diabetic rats. Nutr Res. 2011; 31:479–487. PMID: 21745630.
Article27. Cho JY, Park SC, Kim TW, Kim KS, Song JC, Kim SK, Lee HM, Sung HJ, Park HJ, Song YB, et al. Radical scavenging and anti-inflammatory activity of extracts from Opuntia humifusa Raf. J Pharm Pharmacol. 2006; 58:113–119. PMID: 16393471.
Article28. Kim J, Jho KH, Choi YH, Nam SY. Chemopreventive effect of cactus (Opuntia humifusa) extracts: radical scavenging activity, pro-apoptosis, and anti-inflammatory effect in human colon (SW480) and breast cancer (MCF7) cells. Food Funct. 2013; 4:681–688. PMID: 23435602.
Article29. Yeo JY, Hwang KW, Park SY. Anti-inflammatory effect of neo-lignan isoamericanin A via suppression of NF-κB in liposaccharide-stimulated RAW 264.7 cells. Trop J Pharm Res. 2020; 19:1857–1862.30. Hahm SW, Park J, Son YS. Opuntia humifusa partitioned extracts inhibit the growth of U87MG human glioblastoma cells. Plant Foods Hum Nutr. 2010; 65:247–252. PMID: 20814744.
Article31. Lee SH, Kim HW, Lee MK, Kim YJ, Asamenew G, Cha YS, Kim JB. Phenolic profiling and quantitative determination of common sage (Salvia plebeia R. Br.) by UPLC-DAD-QTOF/MS. Eur Food Res Technol. 2018; 244:1637–1646.
Article32. Kim HW, Lee SH, Yoo SM, Chung MN, Kim JB, Kehraus S, Koenig GM. Identification and quantification of hydroxybenzoyl and hydroxycinnamoyl derivatives from Korean sweet potato cultivars by UPLC-DAD-QToF/MS. J Food Compos Anal. 2021; 100:103905.
Article33. Mosser RE, Maulis MF, Moullé VS, Dunn JC, Carboneau BA, Arasi K, Pappan K, Poitout V, Gannon M. High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Am J Physiol Endocrinol Metab. 2015; 308:E573–E582. PMID: 25628421.
Article34. Antunes LC, Elkfury JL, Jornada MN, Foletto KC, Bertoluci MC. Validation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar rats. Arch Endocrinol Metab. 2016; 60:138–142. PMID: 27191048.35. Nemati M, Zardooz H, Rostamkhani F, Abadi A, Foroughi F. High-fat diet effects on metabolic responses to chronic stress. Arch Physiol Biochem. 2017; 123:182–191. PMID: 28276709.
Article36. Kang J, Lee J, Kwon D, Song Y. Effect of Opuntia humifusa supplementation and acute exercise on insulin sensitivity and associations with PPAR-γ and PGC-1α protein expression in skeletal muscle of rats. Int J Mol Sci. 2013; 14:7140–7154. PMID: 23538842.
Article37. Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003; 26:2442–2450. PMID: 12882876.
Article38. Menzaghi C, Trischitta V, Doria A. Genetic influences of adiponectin on insulin resistance, type 2 diabetes, and cardiovascular disease. Diabetes. 2007; 56:1198–1209. PMID: 17303804.
Article39. Katsiki N, Mantzoros C, Mikhailidis DP. Adiponectin, lipids and atherosclerosis. Curr Opin Lipidol. 2017; 28:347–354. PMID: 28463859.40. Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, Staiger H, Maerker E, Häring H, Stumvoll M. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003; 52:239–243. PMID: 12540592.
Article41. Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine. 2016; 86:100–109. PMID: 27498215.
Article42. Kim EK, Kwon KB, Song MY, Han MJ, Lee JH, Lee YR, Lee JH, Ryu DG, Park BH, Park JW. Flavonoids protect against cytokine-induced pancreatic beta-cell damage through suppression of nuclear factor κB activation. Pancreas. 2007; 35:e1–e9.43. Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007; 380:24–30. PMID: 17343838.
Article44. Jung EY, Yeon SH, Suh HJ. Hypocholesterol effect of Opuntia humifusa extract on high cholesterol diet-induced hypercholesterolemic rats. J Korean Soc Food Sci Nutr. 2014; 43:485–490.45. Baz L, Algarni S, Al-Thepyani M, Aldairi A, Gashlan H. Lycopene improves metabolic disorders and liver injury induced by a hight-fat diet in obese rats. Molecules. 2022; 27:7736. PMID: 36431836.
Article46. Park MK, Lee YJ, Kang ES. Hepatoprotective effect of Cheonnyuncho (Opuntia humifusa) extract in rats treated carbon tetrachloride. Korean J Food Sci Technol. 2005; 37:822–826.47. Boucher P, de Lorgeril M, Salen P, Crozier P, Delaye J, Vallon JJ, Geyssant A, Dante R. Effect of dietary cholesterol on low density lipoprotein-receptor, 3-hydroxy-3-methylglutaryl-CoA reductase, and low density lipoprotein receptor-related protein mRNA expression in healthy humans. Lipids. 1998; 33:1177–1186. PMID: 9930403.
Article48. Jiang H, Yamashita Y, Nakamura A, Croft K, Ashida H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci Rep. 2019; 9:2690. PMID: 30804434.
Article49. Kobori M, Masumoto S, Akimoto Y, Oike H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol Nutr Food Res. 2011; 55:530–540. PMID: 21462320.50. Matboli M, Saad M, Hasanin AH, A Saleh L, Baher W, Bekhet MM, Eissa S. New insight into the role of isorhamnetin as a regulator of insulin signaling pathway in type 2 diabetes mellitus rat model: molecular and computational approach. Biomed Pharmacother. 2021; 135:111176. PMID: 33401224.
Article51. Tundis R, Loizzo MR, Statti GA, Menichini F. Inhibitory effects on the digestive enzyme α-amylase of three Salsola species (Chenopodiaceae) in vitro . Pharmazie. 2007; 62:473–475. PMID: 17663200.52. Yang EI, Lee CH, Che DN, Jang SI, Kim YS. Biological activities of water-soluble polysaccharides from Opuntia humifusa stem in high-fat-diet-fed mice. J Food Biochem. 2019; 43:e12806. PMID: 31353577.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mechanism of Insulin Resistance : Time Dependence of the Development of Insulin Resistance in High Fat Fed Rats
- Insulin Resistance of Skeletal Muscle was Recovered by Leptin Injection in vivo, but not in vitro, in High-fat Diet Fed Rats
- Effect of Exercise Training on Insulin Sensitivity and Intracellular Glucose Metabolism in Skeletal Muscle of High Fat-fed Rats
- Effects of High Fat Diet on Lipolysis in Skeletal Muscle and Adipose Tissue in Rats
- The Inhibitory Effect of Quercetin on Adipose Tissue Inflammation in Mice Fed on a High-fat Diet