Nutr Res Pract.
2024 Aug;18(4):464-478. 10.4162/nrp.2024.18.4.464.
Protective effect of Phyllostachys edulis (Carrière) J. Houz against chronic ethanol-induced cognitive impairment in vivo
- Affiliations
-
- 1Department of Food Science and Nutrition, Pusan National University, Busan 46241, Korea
- 2Department of Food and Nutrition, Kyungsung University, Busan 48434, Korea
- 3Department of Food Science and Nutrition, Gyeongsang National University, Jinju 52725, Korea
- KMID: 2558486
- DOI: http://doi.org/10.4162/nrp.2024.18.4.464
Abstract
- BACKGROUND/OBJECTIVES
Chronic alcohol consumption causes oxidative stress in the body, which may accumulate excessively and cause a decline in memory; problem-solving, learning, and exercise abilities; and permanent damage to brain structure and function. Consequently, chronic alcohol consumption can cause alcohol-related diseases.
MATERIALS/METHODS
In this study, the protective effects of Phyllostachys edulis (Carrière) J. Houz (PE) against alcohol-induced neuroinflammation and cognitive impairment were evaluated using a mouse model. Alcohol (16%, 5 g/kg/day for 6 weeks) and PE (100, 250, and 500 mg/kg/day for 21 days) were administered intragastrically to mice.
RESULTS
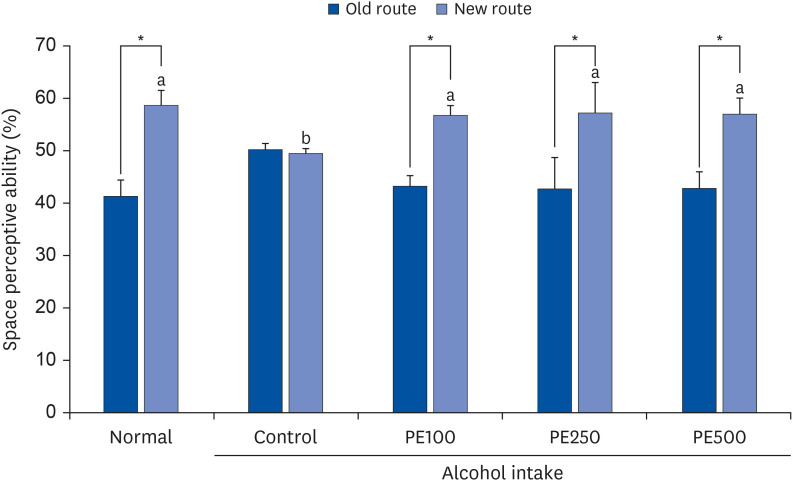
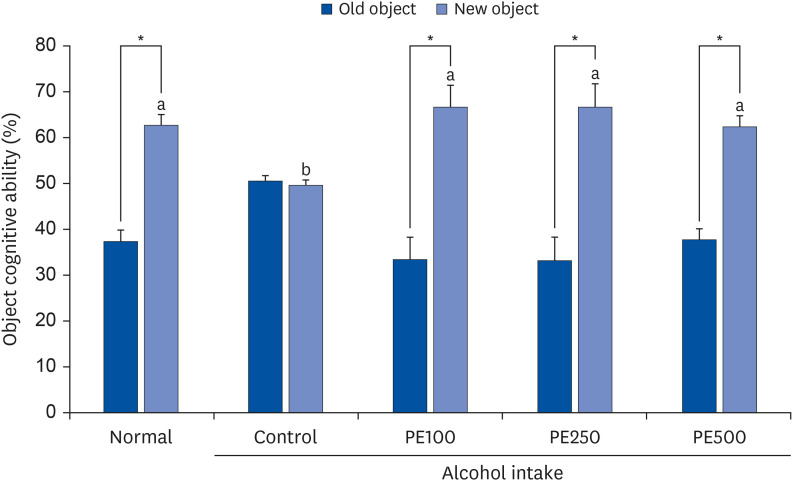
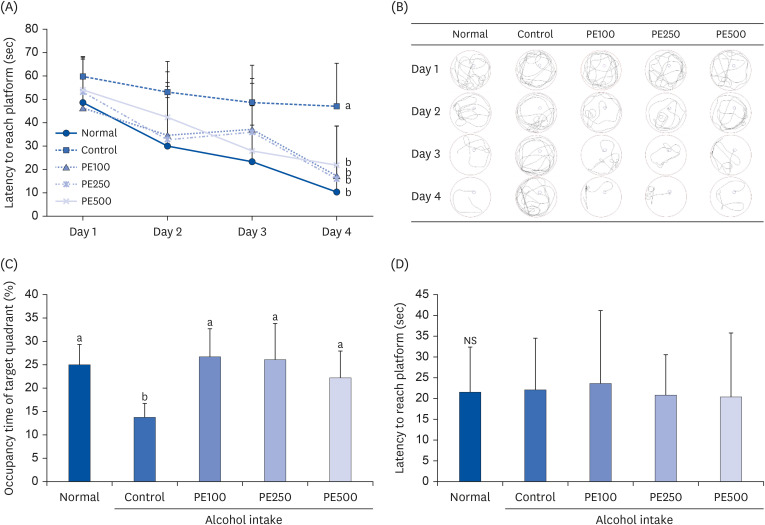
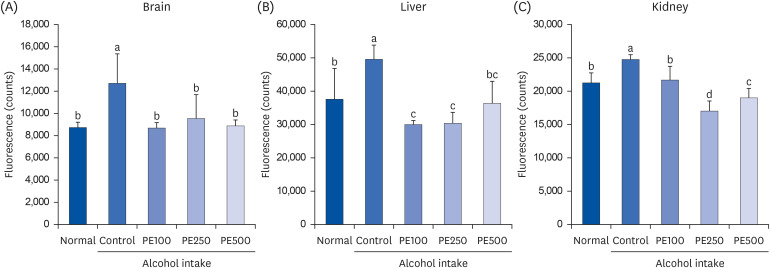
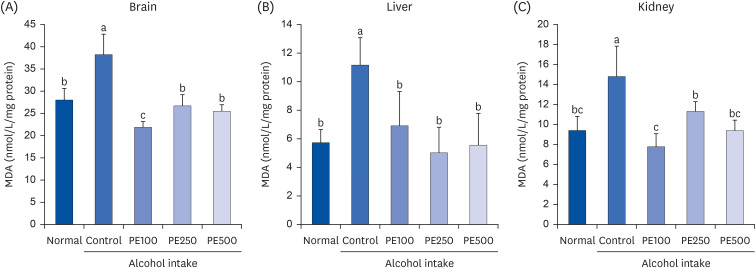
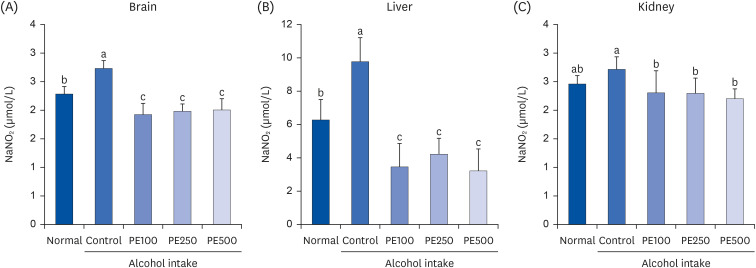
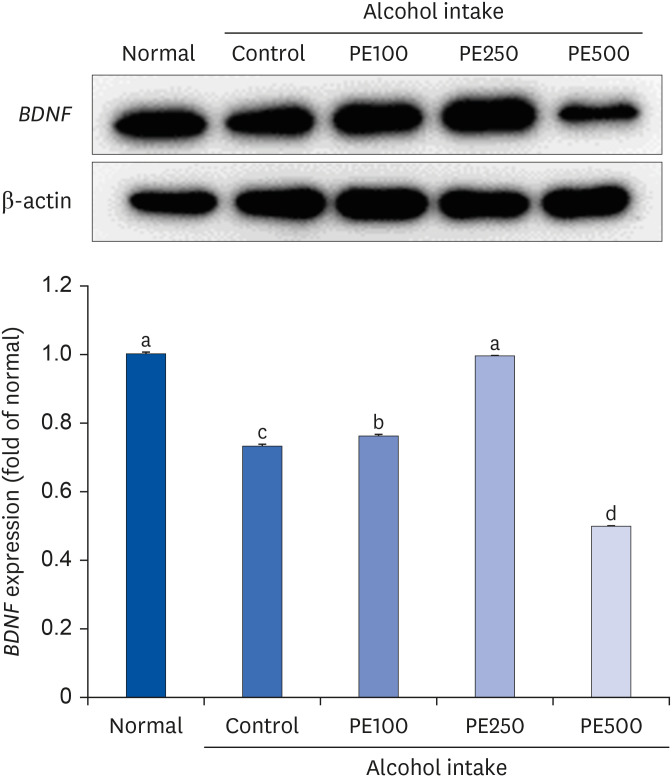
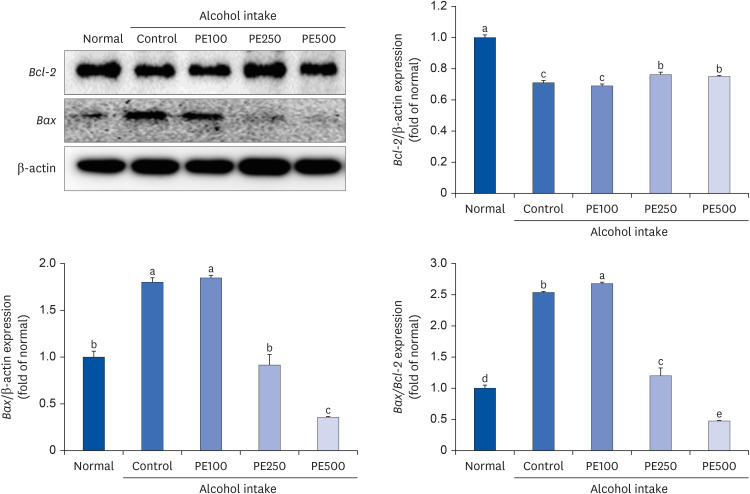
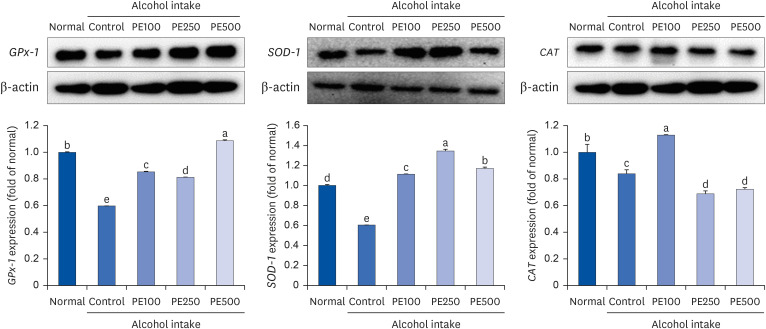
PE showed a protective effect against memory deficits and cognitive dysfunction caused by alcohol consumption, confirmed through behavioral tests such as the T-maze, object recognition, and Morris water maze tests. Additionally, PE attenuated oxidative stress by reducing lipid oxidation, nitric oxide, and reactive oxygen species levels in the mice’s brains, livers, and kidneys. Improvement of neurotrophic factors and downregulation of apoptosis-related proteins were confirmed in the brains of mice fed low and medium concentrations of PE. Additionally, expression of antioxidant enzyme-related proteins GPx-1 and SOD-1 was enhanced in the liver of PE-treated mice, related to their inhibitory effect on oxidative stress.
CONCLUSION
This suggests that PE has both neuroregenerative and antioxidant effects. Collectively, these behavioral and histological results confirmed that PE could improve alcohol-induced cognitive deficits through brain neurotrophic and apoptosis protection and modulation of oxidative stress.
Figure
Reference
-
1. Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004; 74:223–234. PMID: 15194200.
Article2. Zahr NM, Kaufman KL, Harper CG. Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol. 2011; 7:284–294. PMID: 21487421.3. Butterworth RF. Hepatic encephalopathy--a serious complication of alcoholic liver disease. Alcohol Res Health. 2003; 27:143–145. PMID: 15303624.4. Cheon YH, Joe KH, Kim DJ. Alcohol-related dementia. J Korean Geriatr Psychiatry. 2012; 16:89–96.5. Korea Forest Research Institute. Encyclopedia of Bamboos in Korea; New Research Report 7. Seoul: Ukgo Publishing;2005. p. 203.6. Cho WD. Studies on the functional components extracted from bamboo. Coop Manag Inst. 2000; 23:95–108.7. Cho E, Kim S, Na I, Kim DC, In MJ, Chae HJ. Antioxidant and anticoagulant activities of water and ethanol extracts of Phyllostachys pubescence leaf produced in Geoje. J Appl Biol Chem. 2010; 53:170–173.
Article8. Shibata M, Sato F, Takeshita K, Otani K. Pharmacological studies on bamboo grass Sasa-albomarginata 5. Combined effects of the extract F-D with vitamin C. Shoyakugaku Zasshi. 1980; 34:274–279.9. Heo J. DongUiBoGam-DongUiBoGam Korean Translate Committee. Seoul: Bubinmunhwasa;1990. p. 192–1392.10. Tundis R, Augimeri G, Vivacqua A, Romeo R, Sicari V, Bonofiglio D, Loizzo MR. Anti-inflammatory and antioxidant effects of leaves and sheath from bamboo (Phyllostacys edulis J. Houz). Antioxidants. 2023; 12:1239. PMID: 37371969.
Article11. Rajebhosale VA, Burte RG, Toro VA. Nutritive value of bamboo (Dendrocalamus calostachyus) leaves for crossbred calves. Indian J Anim Nutr. 1998; 15:58–60.12. Tsunoda S, Yamamoto K, Sakamoto S, Inoue H, Nagasawa H. Effects of Sasa Health, extract of bamboo grass leaves, on spontaneous mammary tumourigenesis in SHN mice. Anticancer Res. 1998; 18:153–158. PMID: 9568070.13. Michiko F. Difference between bamboo shoots and vegetables in thermal disintegration of tissues and polysaccharides fractionated by successive extraction. J Food Sci. 1990; 55:739–745.14. Montgomery KC. A test of two explanations of spontaneous alternation. J Comp Physiol Psychol. 1952; 45:287–293. PMID: 14946277.
Article15. Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012; 13:93–110. PMID: 22160349.
Article16. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11:47–60. PMID: 6471907.
Article17. Muhammad T, Ali T, Ikram M, Khan A, Alam SI, Kim MO. Melatonin rescue oxidative stress-mediated neuroinflammation/neurodegeneration and memory impairment in scopolamine-induced amnesia mice model. J Neuroimmune Pharmacol. 2019; 14:278–294. PMID: 30478761.
Article18. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358. PMID: 36810.19. Schmidt HH, Pollock JS, Nakane M, Förstermann U, Murad F. Ca2+/calmodulin-regulated nitric oxide synthases. Cell Calcium. 1992; 13:427–434. PMID: 1380405.20. Gerridzen IJ, Hertogh CM, Depla MF, Veenhuizen RB, Verschuur EM, Joling KJ. Neuropsychiatric symptoms in people with Korsakoff syndrome and other alcohol-related cognitive disorders living in specialized long-term care facilities: prevalence, severity, and associated caregiver distress. J Am Med Dir Assoc. 2018; 19:240–247. PMID: 29079031.21. Huang WJ, Zhang X, Chen WW. Association between alcohol and Alzheimer’s disease. Exp Ther Med. 2016; 12:1247–1250. PMID: 27588045.22. Signorini-Allibe N, Gonthier B, Lamarche F, Eysseric H, Barret L. Chronic consumption of ethanol leads to substantial cell damage in cultured rat astrocytes in conditions promoting acetaldehyde accumulation. Alcohol Alcohol. 2005; 40:163–171. PMID: 15767272.
Article23. Koop DR. Alcohol metabolism’s damaging effects on the cell: a focus on reactive oxygen generation by the enzyme cytochrome P450 2E1. Alcohol Res Health. 2006; 29:274–280. PMID: 17718406.24. Climent E, Pascual M, Renau-Piqueras J, Guerri C. Ethanol exposure enhances cell death in the developing cerebral cortex: role of brain-derived neurotrophic factor and its signaling pathways. J Neurosci Res. 2002; 68:213–225. PMID: 11948666.
Article25. Kweon MH, Hwang HJ, Sung HC. Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis). J Agric Food Chem. 2001; 49:4646–4655. PMID: 11600002.
Article26. Kim EY, Lee MJ, Song YO, Moon GS. Effect of maengjong-juk (Phyllostachys pubescens) extract coated rice diet on antioxidative system of C57BL/6 mice fed atherogenic diet. Korean J Community Nutr. 2004; 9:536–544.27. Mamiya T, Kise M, Morikawa K. Ferulic acid attenuated cognitive deficits and increase in carbonyl proteins induced by buthionine-sulfoximine in mice. Neurosci Lett. 2008; 430:115–118. PMID: 18061347.
Article28. Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006; 1:848–858. PMID: 17406317.29. Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006; 1:1306–1311. PMID: 17406415.
Article30. Alderson RF, Alterman AL, Barde YA, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990; 5:297–306. PMID: 2169269.
Article31. Peregud DI, Baronets VY, Terebilina NN, Gulyaeva NV. Role of BDNF in neuroplasticity associated with alcohol dependence. Biochemistry (Mosc). 2023; 88:404–416. PMID: 37076286.32. MacLennan AJ, Lee N, Walker DW. Chronic ethanol administration decreases brain-derived neurotrophic factor gene expression in the rat hippocampus. Neurosci Lett. 1995; 197:105–108. PMID: 8552271.
Article33. Abrahao KP, Salinas AG, Lovinger DM. Alcohol and the brain: neuronal molecular targets, synapses, and circuits. Neuron. 2017; 96:1223–1238. PMID: 29268093.34. Ceballos N, Sharma S. Risk and resilience: the role of brain-derived neurotrophic factor in alcohol use disorder. AIMS Neurosci. 2016; 3:398–432.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk and Protective Factors for the Onset of Cognitive Impairment in Korea: A 10-Year Longitudinal Panel Study
- Effect of Glycyrrhizic Acid on Scopolamine-Induced Cognitive Impairment in Mice
- Effect of Environmental Enrichment on Cognitive Impairment-induced by Ethanol Exposure in Adolescent Rat
- Serochemical and Histopathological Observations on the Effect of Malotilate in Chronic Liver Injury Induced by Carbon Tetrachloride with or without Ethanol
- Inhibitory effect of an ethanol extract mixture of Aralia elata leaf, Chaenomeles sinensis fruit and Glycyrrhizae radix on Aβ (25–35)-induced memory impairment