Brain Tumor Res Treat.
2024 Jul;12(3):153-161. 10.14791/btrt.2024.0027.
Trends in Developing Extracellular Vesicle-Based Therapeutics
- Affiliations
-
- 1Department of Life Sciences, Pohang University of Science and Technology (POSTECH), Pohang, Korea
- KMID: 2558433
- DOI: http://doi.org/10.14791/btrt.2024.0027
Abstract
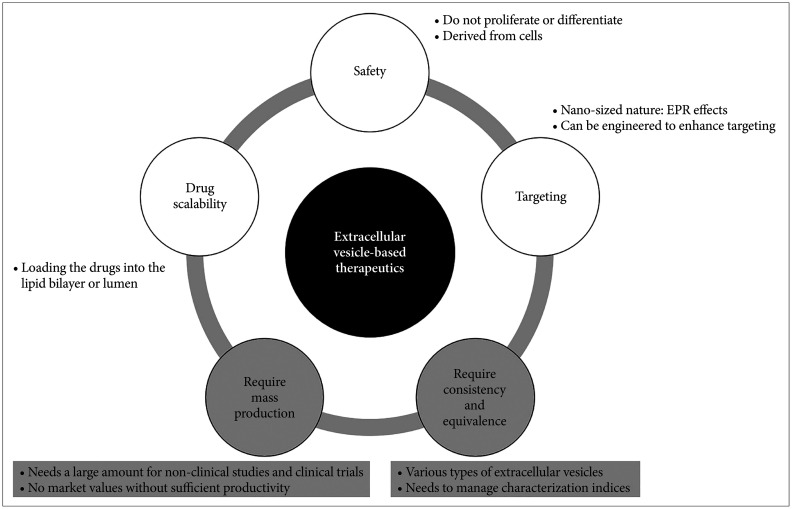
- Extracellular vesicles are nano-sized vesicles surrounded by lipid bilayers, and all cells release them to the extracellular environment for communication. Extracellular vesicles consist of molecules with various biological activities and can play essential roles as therapeutics, so they attract much attention as next-generation modalities to treat various diseases. As extracellular vesicles are cell-derived nanovesicles, they are favorable to be developed as therapeutics, but they also have limitations. In addition, there are a number of things to consider in terms of manufacturing, quality control, non-clinical studies, and clinical trials during the development of extracellular vesicle-based therapeutics. Meanwhile, as much attention has been paid to the potentials of extracellular vesicles as therapeutics, many biopharmaceutical companies are trying to develop extracellular vesicle-based therapeutics. This review will introduce the advantages and limitations of extracellular vesicles as therapeutics. In addition, it will cover things to consider during developing extracellular vesicle-based therapeutics and development cases of extracellular vesicle-based therapeutics.
Keyword
Figure
Reference
-
1. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009; 9:581–593. PMID: 19498381.2. Kim DK, Lee J, Kim SR, Choi DS, Yoon YJ, Kim JH, et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics. 2015; 31:933–939. PMID: 25388151.3. Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol. 2015; 40:97–104. PMID: 25704309.4. Gill S, Catchpole R, Forterre P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol Rev. 2019; 43:273–303. PMID: 30476045.5. Gho YS, Lee C. Emergent properties of extracellular vesicles: a holistic approach to decode the complexity of intercellular communication networks. Mol Biosyst. 2017; 13:1291–1296. PMID: 28488707.6. Hirschberg Y, Valle-Tamayo N, Dols-Icardo O, Engelborghs S, Buelens B, Vandenbroucke RE, et al. Proteomic comparison between non-purified cerebrospinal fluid and cerebrospinal fluid-derived extracellular vesicles from patients with Alzheimer’s, Parkinson’s and Lewy body dementia. J Extracell Vesicles. 2023; 12:e12383. PMID: 38082559.7. Cross T, Øvstebø R, Brusletto BS, Trøseid AS, Olstad OK, Aspelin T, et al. RNA profiles of tear fluid extracellular vesicles in patients with dry eye-related symptoms. Int J Mol Sci. 2023; 24:15390. PMID: 37895069.8. Park JO, Choi DY, Choi DS, Kim HJ, Kang JW, Jung JH, et al. Identification and characterization of proteins isolated from microvesicles derived from human lung cancer pleural effusions. Proteomics. 2013; 13:2125–2134. PMID: 23585444.9. Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom Rev. 2015; 34:474–490. PMID: 24421117.10. Lötvall J, Rajendran L, Gho YS, Thery C, Wauben M, Raposo G, et al. The launch of Journal of Extracellular Vesicles (JEV), the official journal of the International Society for Extracellular Vesicles – about microvesicles, exosomes, ectosomes and other extracellular vesicles. J Extracell Vesicles. 2012; 1:18514.11. Korean Society for Extracellular Vesicles: Main page. Seoul: Korean Society for Extracellular Vesicles;Accessed April 24, 2024. at https://ksev.or.kr.12. National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety. Guideline on quality, non-clinical and clinical assessment of extracellular vesicles therapy products (guideline-0917-02) (partially amended on December 21, 2023). Available at: https://www.mfds.go.kr/brd/m_1060/down.do?brd_id=data0011&seq=15422&data_tp=A&file_seq=3.13. Choi DS, Kim DK, Kim YK, Gho YS. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 2013; 13:1554–1571. PMID: 23401200.14. Mayr M, Grainger D, Mayr U, Leroyer AS, Leseche G, Sidibe A, et al. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ Cardiovasc Genet. 2009; 2:379–388. PMID: 20031610.15. Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019; 51:1–12.16. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014; 14:195–208. PMID: 24566916.17. Global exosome therapeutics market – industry trends and forecast to 2030. Pune: Data Bridge Market Research;2023. Accessed April 24, 2024. at https://www.databridgemarketresearch.com/reports/global-exosome-therapeutic-market.18. Ben-David U, Arad G, Weissbein U, Mandefro B, Maimon A, Golan-Lev T, et al. Aneuploidy induces profound changes in gene expression, proliferation and tumorigenicity of human pluripotent stem cells. Nat Commun. 2014; 5:4825. PMID: 25198699.19. Buganim Y, Markoulaki S, van Wietmarschen N, Hoke H, Wu T, Ganz K, et al. The developmental potential of iPSCs is greatly influenced by reprogramming factor selection. Cell Stem Cell. 2014; 15:295–309. PMID: 25192464.20. Zhang K, Cheng K. Stem cell-derived exosome versus stem cell therapy. Nat Rev Bioeng. 2023; 1:608–609.21. Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers. Front Oncol. 2013; 3:211. PMID: 23967403.22. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017; 17:20–37. PMID: 27834398.23. Cecchin R, Troyer Z, Witwer K, Morris KV. Extracellular vesicles: the next generation in gene therapy delivery. Mol Ther. 2023; 31:1225–1230. PMID: 36698310.24. Song H, Chen X, Hao Y, Wang J, Xie Q, Wang X. Nanoengineering facilitating the target mission: targeted extracellular vesicles delivery systems design. J Nanobiotechnology. 2022; 20:431. PMID: 36175866.25. Fu S, Wang Y, Xia X, Zheng JC. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact. 2020; 20:100261.26. Grangier A, Branchu J, Volatron J, Piffoux M, Gazeau F, Wilhelm C, et al. Technological advances towards extracellular vesicles mass production. Adv Drug Deliv Rev. 2021; 176:113843. PMID: 34147532.27. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019; 9:19. PMID: 30815248.28. Paolini L, Monguió-Tortajada M, Costa M, Antenucci F, Barilani M, Clos-Sansalvador M, et al. Large-scale production of extracellular vesicles: report on the “massivEVs” ISEV workshop. J Extracell Biol. 2022; 1:e63. PMID: 38939213.29. Bellani CF, Ajeian J, Duffy L, Miotto M, Groenewegen L, Connon CJ. Scale-up technologies for the manufacture of adherent cells. Front Nutr. 2020; 7:575146. PMID: 33251241.30. Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo AB, et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011; 9:47. PMID: 21513579.31. Patel DB, Santoro M, Born LJ, Fisher JP, Jay SM. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol Adv. 2018; 36:2051–2059. PMID: 30218694.32. Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013; 7:7698–7710. PMID: 24004438.33. Ministry of Food and Drug Safety. Regulation on approval and review of biological products (notice no. 2024-31) (partially amended and enforced on June 25, 2024). Available at: https://www.mfds.go.kr/brd/m_207/down.do?brd_id=data0008&seq=14984&data_tp=A&file_seq=2.34. National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety. Guideline on quality, non-clinical and clinical assessment of extracellular vesicles therapy products (guideline-0917-01) (established on December 27, 2018). Available at: https://www.nifds.go.kr/brd/m_15/view.do?seq=12625.35. National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety. [Information collection for quality and non-clinical assessment of stem cell-derived extracellular vesicles therapy products]. Seoul: Jinhan M&B;2022. Korean.36. National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety. Guideline on eligibility determination for donors of cell therapy products (guideline 0311-02) (partially amended on June 7, 2021). Available at: https://www.mfds.go.kr/brd/m_1060/view.do?seq=14860&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=90.37. National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety. Guideline for cell bank evaluation of cell therapy products (guideline 1141-02) (partially amended on October 31, 2023). Available at: https://www.mfds.go.kr/brd/m_1060/view.do?seq=15379.38. Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2022; 9:811971. PMID: 35071216.39. Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015; 4:30087. PMID: 26725829.40. Quah BJ, O’Neill HC. Mycoplasma contaminants present in exosome preparations induce polyclonal B cell responses. J Leukoc Biol. 2007; 82:1070–1082. PMID: 17698916.41. Ministry of Food and Drug Safety. Standard for stability study of pharmaceuticals (notice 2019-132) (partially amended and enforced on December 17, 2019). Available at: https://www.mfds.go.kr/brd/m_211/view.do?seq=14421.42. Ministry of Food and Drug Safety. Standard for toxicity study of pharmaceuticals (notice no. 2022-18) (partially amended and enforced on March 2, 2022). Available at: https://www.law.go.kr/LSW/admRulLsInfoP.do?chrClsCd=&admRulSeq=2100000209652.43. BEXSERO (Meningococcal group B vaccine) suspension, for intramuscular injection. Durham: GSK;2023. Accessed April 24, 2024. at https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Bexsero/pdf/BEXSERO.PDF.44. GSK delivers strong 2023 performance and upgrades growth outlooks. Brentford: GSK;2024. Accessed April 24, 2024. at https://www.gsk.com/media/10928/fy-2023-results-announcement.pdf.45. A first-in-human study of CDK-002 (exoSTING) in subjects with advanced/metastatic, recurrent, injectable solid tumors. Cambridge: Codiak BioSciences;Accessed April 24, 2024. at https://clinicaltrials.gov/study/NCT04592484.46. Codiak announces platform-validating clinical data from phase 1 trials of ExoSTING and ExoIL-12. New York: Markets Insider;2022. Accessed April 24, 2024. at https://markets.businessinsider.com/news/stocks/codiak-announces-platform-validating-clinical-data-from-phase-1-trials-of-exosting-and-exoil-12-1031559977.47. A phase 1/2a study of CDK-003 in patients with cutaneous T-cell lymphoma (CTCL). Bethesda: U.S. National Library of Medicine;2022. Accessed April 24, 2024. at https://clinicaltrials.gov/study/NCT05156229.48. A study of exoASO-STAT6 (CDK-004) in patients with advanced hepatocellular carcinoma (HCC) and patients with liver metastases from either primary gastric cancer or colorectal cancer (CRC). Bethesda: U.S. National Library of Medicine;2023. Accessed April 24, 2024. at https://clinicaltrials.gov/study/NCT05375604.49. Jazz Pharmaceuticals and Codiak BioSciences announce strategic collaboration to research, develop and commercialize engineered exosomes to create therapies for hard-to-treat cancers. Dublin: Jazz Pharmaceuticals;2019. Accessed April 24, 2024. at https://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-and-codiak-biosciences-announce-strategic-0.50. Codiak files for bankruptcy after exosome-focused biotech unable to satisfy ‘financial needs’. New York: Fierce Biotech;2023. Accessed April 24, 2024. at https://www.fiercebiotech.com/biotech/codiak-biosciences-files-bankruptcy-after-unable-satisfy-financial-needs.51. Evox Therapeutics enters into agreement to advance next generation exosome-delivered AAV gene therapy for the treatment of heart disease. Oxford: Evox Therapeutics;2023. Accessed April 24, 2024. at https://www.evoxtherapeutics.com/evox-therapeutics-enters-into-agreement-to-advance-next-generation-exosome-delivered-aav-gene-therapy-for-the-treatment-of-heart-disease.52. Li X, La Salvia S, Liang Y, Adamiak M, Kohlbrenner E, Jeong D, et al. Extracellular vesicle-encapsulated adeno-associated viruses for therapeutic gene delivery to the heart. Circulation. 2023; 148:405–425. PMID: 37409482.53. Evox Therapeutics acquires exosome AAV technology and intellectual property. Oxford: Evox Therapeutics;2023. Accessed April 24, 2024. at https://www.evoxtherapeutics.com/evox-therapeutics-acquires-exosome-aav-technology-and-intellectual-property.54. Pipeline. Woburn: AEGLE Therapeutics. Accessed April 24, 2024. at https://aegletherapeutics.com/pipeline.55. MSC EVs in dystrophic epidermolysis bullosa. Bethesda: U.S. National Library of Medicine;2024. Accessed April 24, 2024. at https://clinicaltrials.gov/study/NCT04173650.56. Safety of extracellular vesicles for burn wounds. Bethesda: U.S. National Library of Medicine;2023. updated Mar 29, 2024. Accessed April 24, 2024. at https://clinicaltrials.gov/study/NCT05078385.57. AEGLE Therapeutics Corp. announces positive data for the first patient in a phase 1/2a clinical trial dosed with AGLE-102™, a novel extracellular vesicle therapy. Woburn: AEGLE Therapeutics;2024. Accessed April 24, 2024. at https://www.prnewswire.com/news-releases/aegle-therapeutics-corp-announces-positive-data-for-the-first-patient-in-a-phase-12a-clinical-trial-dosed-with-agle-102-a-novel-extracellular-vesicle-therapy-302027568.html.58. ExoFlo™. Austin: Direct Biologics;Accessed April 24, 2024. at https://directbiologics.com/exoflo.59. Extracellular vesicle infusion treatment for COVID-19 associated ARDS (EXIT-COVID19). Bethesda: U.S. National Library of Medicine;2020. updated Feb 13, 2024. Accessed April 24, 2024. at https://clinicaltrials.gov/study/NCT04493242.60. Lightner AL, Sengupta V, Qian S, Ransom JT, Suzuki S, Park DJ, et al. Bone marrow mesenchymal stem cell-derived extracellular vesicle infusion for the treatment of respiratory failure from COVID-19: a randomized, placebo-controlled dosing clinical trial. Chest. 2023; 164:1444–1453. PMID: 37356708.61. Study of ExoFlo for the treatment of medically refractory Crohn’s disease. Bethesda: U.S. National Library of Medicine;2023. updated Feb 12, 2024. Accessed April 24, 2024. at https://clinicaltrials.gov/study/NCT05130983.62. Study of ExoFlo for the treatment of medically refractory ulcerative colitis. Bethesda: U.S. National Library of Medicine;2022. updated Feb 12, 2024. Accessed April 24, 2024. at https://clinicaltrials.gov/study/NCT05176366.63. Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016; 7:12277. PMID: 27447450.64. Choi H, Kim Y, Mirzaaghasi A, Heo J, Kim YN, Shin JH, et al. Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality. Sci Adv. 2020; 6:eaaz6980. PMID: 32285005.65. Kim S, Lee SA, Yoon H, Kim MY, Yoo JK, Ahn SH, et al. Exosome-based delivery of super-repressor IκBα ameliorates kidney ischemia-reperfusion injury. Kidney Int. 2021; 100:570–584. PMID: 34051264.66. Ililas biologics and Brexogen’s exosome-based drug development trials. Seoul: HitNews;2023. Accessed April 24, 2024. at http://www.hitnews.co.kr/news/articleView.html?idxno=49243.67. BREXOGEN. Platform - BG Platform. Seoul: BREXOGEN;Accessed April 24, 2024. at http://brexogen.com/m21.php.68. Kim J, Lee SK, Jung M, Jeong SY, You H, Won JY, et al. Extracellular vesicles from IFN-γ-primed mesenchymal stem cells repress atopic dermatitis in mice. J Nanobiotechnology. 2022; 20:526. PMID: 36496385.69. A study to investigate the safety, tolerability, and efficacy of BxC-I17e single-dose SC injection in patients with moderate to severe atopic dermatitis. Bethesda: U.S. National Library of Medicine;2023. Accessed April 24, 2024. at https://clinicaltrials.gov/study/NCT06055361.70. Lau HC, Han DW, Park J, Lehner E, Kals C, Arzt C, et al. GMP-compliant manufacturing of biologically active cell-derived vesicles produced by extrusion technology. J Extracell Biol. 2022; 1:e70. PMID: 38938599.71. Caravan Biologix, Inc. collaborates with MDimune on cell-derived nanovesicles for cancer. San Francisco: Business Wire;Accessed April 24, 2024. at https://www.businesswire.com/news/home/20220915005157/en/Caravan-Biologix-Inc.-Collaborates-with-MDimune-on-Cell-Derived-NanoVesicles-for-Cancer.72. MDimune wins bronze at 2023 Edison Awards. Seoul: NewsWire;2023. Accessed April 24, 2024. at https://www.newswire.co.kr/newsRead.php?no=965697.73. GNV. Incheon: SL Bigen;Accessed April 24, 2024. at http://slbigen.com/?page_id=532.74. Go G, Lee J, Choi DS, Kim SS, Gho YS. Extracellular vesicle-mimetic ghost nanovesicles for delivering anti-inflammatory drugs to mitigate Gram-negative bacterial outer membrane vesicle-induced systemic inflammatory response syndrome. Adv Healthc Mater. 2019; 8:e1801082. PMID: 30549424.75. REX101. Incheon: SL Bigen;Accessed April 24, 2024. at http://slbigen.com/?page_id=558/#.76. Won S, Lee C, Bae S, Lee J, Choi D, Kim MG, et al. Mass-produced Gram-negative bacterial outer membrane vesicles activate cancer antigen-specific stem-like CD8+ T cells which enables an effective combination immunotherapy with anti-PD-1. J Extracell Vesicles. 2023; 12:e12357. PMID: 37563797.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Metaverse for Healthcare: Trends, Applications, and Future Directions of Digital Therapeutics for Urology
- Strategies to Enhance Extracellular Vesicle Production
- Seminal Vesicle Infection of Zinner Syndrome Misdiagnosed for Neoplasm
- Engineering of Extracellular Vesicles Based on Payload Changes for Tissue Regeneration
- 1 case of seminal vesicle cyst