Ann Surg Treat Res.
2024 Aug;107(2):91-99. 10.4174/astr.2024.107.2.91.
Multivariable linear model for predicting graft weight based on 3-dimensional volumetry in regards to body weight change of living liver donor: an observational cohort study
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2558385
- DOI: http://doi.org/10.4174/astr.2024.107.2.91
Abstract
- Purpose
The purpose of this study is to build a prediction model for estimating graft weight about different graft volumetry methods combined with other variables.
Methods
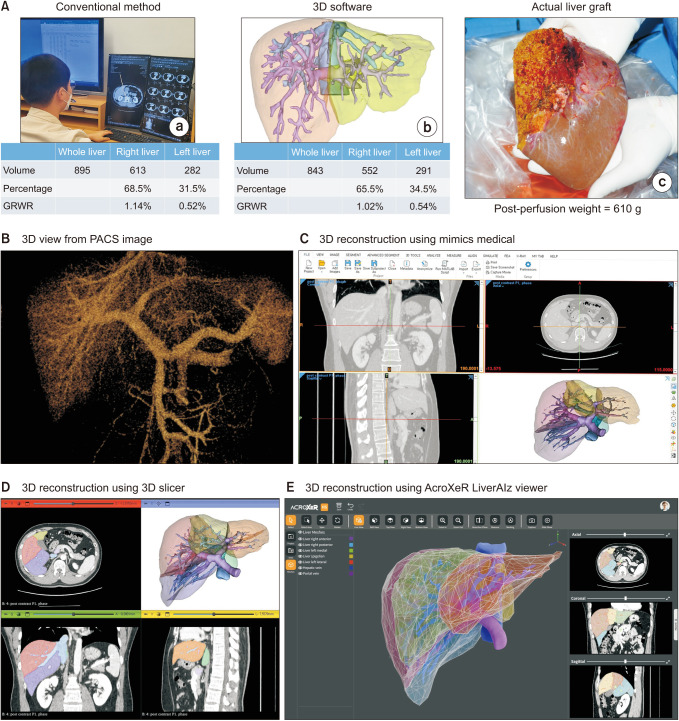
Donors who underwent living-donor right hepatectomy from March 2021 to March 2023 were included. Estimated graft volume measured by conventional method and 3-dimensional (3D) software were collected as well as the actual graft weight. Linear regression was used to build a prediction model. Donor groups were divided according to the 3D volumetry of <700 cm3 , 700–899 cm3 , and ≥900 cm3 to compare the performance of different models.
Results
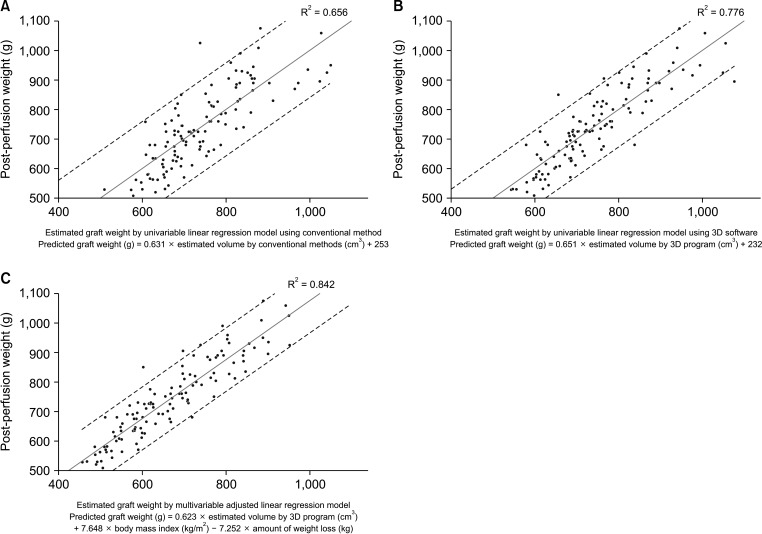
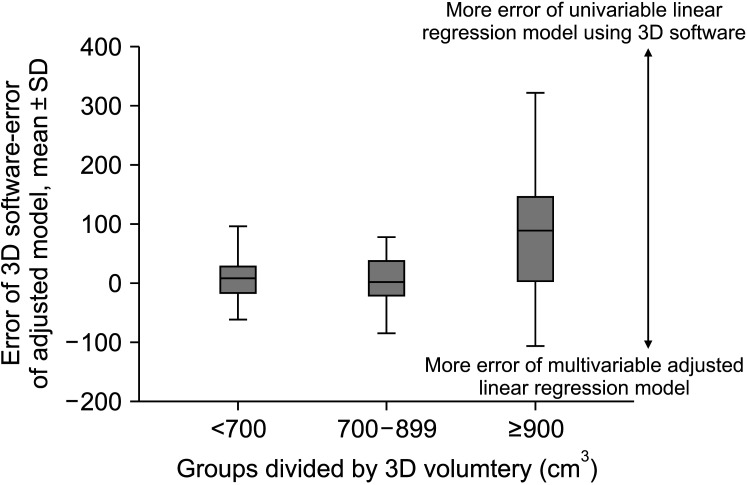
A total of 119 donors were included. Conventional volumetry showed R2 of 0.656 (P < 0.001) while 3D software showed R2 of 0.776 (P < 0.001). The R2 of the multivariable model was 0.842 (P < 0.001) including for 3D volume (β = 0.623, P < 0.001), body mass index (β = 7.648, P < 0.001), and amount of weight loss (β = –7.252, P < 0.001). The median errors between different models and actual graft weight did not differ in donor groups (<700 and 700–899 cm3 ), while the median error of univariable linear model using 3D software (122.5; interquartile range [IQR], 61.5–179.8) was significantly higher than multivariable-adjusted linear model (41.5; IQR, 24.8–69.8; P = 0.003) in donors with estimated graft weight ≥900 cm3 .
Conclusion
The univariable 3D volumetry model showed an acceptable outcome for donors with an estimated graft volume <900 cm3 . For donors with an estimated graft volume ≥900 cm3 , the multivariable-adjusted linear model showed higher accuracy.
Figure
Reference
-
1. Ikegami T, Balci D, Jung DH, Kim JM, Quintini C. Living donor liver transplantation in small-for-size setting. Int J Surg. 2020; 82S:134–137. PMID: 32738547.2. Ikegami T, Onda S, Furukawa K, Haruki K, Shirai Y, Gocho T. Small-for-size graft, small-for-size syndrome and inflow modulation in living donor liver transplantation. J Hepatobiliary Pancreat Sci. 2020; 27:799–809. PMID: 32897590.3. Jeong WK. Clinical implication of hepatic volumetry for living donor liver transplantation. Clin Mol Hepatol. 2018; 24:51–53. PMID: 29580036.4. Jeong JG, Choi S, Kim YJ, Lee WS, Kim KG. Deep 3D attention CLSTM U-Net based automated liver segmentation and volumetry for the liver transplantation in abdominal CT volumes. Sci Rep. 2022; 12:6370. PMID: 35430594.5. Park R, Lee S, Sung Y, Yoon J, Suk HI, Kim H, et al. Accuracy and efficiency of right-lobe graft weight estimation using deep-learning-assisted CT volumetry for living-donor liver transplantation. Diagnostics (Basel). 2022; 12:590. PMID: 35328143.6. Rhu J, Choi GS, Kim JM, Kwon CHD, Joh JW. Risk factors associated with surgical morbidities of laparoscopic living liver donors. Ann Surg. 2023; 278:96–102. PMID: 36994737.7. Hwang S, Lee SG, Jang SJ, Cho SH, Kim KH, Ahn CS, et al. The effect of donor weight reduction on hepatic steatosis for living donor liver transplantation. Liver Transpl. 2004; 10:721–725. PMID: 15162464.8. Trakroo S, Bhardwaj N, Garg R, Modaresi Esfeh J. Weight loss interventions in living donor liver transplantation as a tool in expanding the donor pool: a systematic review and meta-analysis. World J Gastroenterol. 2021; 27:3682–3692. PMID: 34239278.9. Choi J, Choi Y, Hong SY, Suh S, Hong K, Han ES, et al. Changes in indices of steatosis and fibrosis in liver grafts of living donors after weight reduction. Front Surg. 2022; 9:827526. PMID: 35592121.10. Oh N, Kim JH, Rhu J, Jeong WK, Choi GS, Kim JM, et al. 3D auto-segmentation of biliary structure of living liver donors using magnetic resonance cholangiopancreatography for enhanced preoperat ive planning. Int J Surg. 2024; 110:1975–1982. PMID: 38668656.11. Oh N, Kim JH, Rhu J, Jeong WK, Choi GS, Kim JM, et al. Automated 3D liver segmentation from hepatobiliary phase MRI for enhanced preoperative planning. Sci Rep. 2023; 13:17605. PMID: 37848662.12. Yoshizumi T, Itoh S, Shimokawa M, Inokuchi S, Harada N, Takeishi K, et al. Simultaneous splenectomy improves outcomes after adult living donor liver transplantation. J Hepatol. 2021; 74:372–379. PMID: 32827564.13. Yang X, Lee MR, Yang JD. A new formula for estimation of standard liver volume using liver height and thoracic width. Ann Surg Treat Res. 2022; 103:47–52. PMID: 35919114.14. Rhu J, Kim JM, Jeong WK, Choi GS, Joh JW. Venous outflow congestion is related to poor recurrence-free survival of living donor liver transplantation recipients with hepatocellular carcinoma: a retrospective study. Transpl Int. 2021; 34:272–280. PMID: 33253442.15. Lim C, Turco C, Balci D, Savier E, Goumard C, Perdigao F, et al. Auxiliary liver transplantation for cirrhosis: from APOLT to RAPID. A scoping review. Ann Surg. 2022; 275:551–559. PMID: 34913893.16. Rhu J, Choi GS, Kim MS, Kim JM, Joh JW. Image guidance using two-dimensional illustrations and three-dimensional modeling of donor anatomy during living donor hepatectomy. Clin Transplant. 2021; 35:e14164. PMID: 33222255.17. Rhu J, Kim MS, Choi GS, Kim JM, Kwon CH, Joh JW. Laparoscopic living donor right hepatectomy regarding the anatomical variation of the portal vein: a propensity score-matched analysis. Liver Transpl. 2021; 27:984–996. PMID: 33711190.18. Rhu J, Lim S, Kang D, Cho J, Lee H, Choi GS, et al. Virtual reality education program including three-dimensional individualized liver model and education videos: a pilot case report in a patient with hepatocellular carcinoma. Ann Hepatobiliary Pancreat Surg. 2022; 26:285–288. PMID: 35473767.19. Rhu J, Choi GS, Kim JM, Kwon CH, Joh JW. Complete transition from open surgery to laparoscopy: 8-year experience with more than 500 laparoscopic living donor hepatectomies. Liver Transpl. 2022; 28:1158–1172. PMID: 35138684.20. Park S, Choi GS, Kim JM, Lee S, Joh JW, Rhu J. 3D printing model of abdominal cavity of liver transplantation recipient to prevent large-for-size syndrome. Int J Bioprint. 2022; 8:609. PMID: 36404778.21. Rhu J, Kim MS, Kim S, Choi GS, Kim JM, Joh JW. Application of three-dimensional printing for intraoperative guidance during liver resection of a hepatocellular carcinoma with sophisticated location. Ann Hepatobiliary Pancreat Surg. 2021; 25:265–269. PMID: 34053930.22. Rhu J, Kim MS, Choi GS, Jeong WK, Kim JM, Joh JW. A novel technique for bile duct division during laparoscopic living donor hepatectomy to overcome biliary complications in liver transplantation recipients: "cut and clip" rather than "clip and cut". Transplantation. 2021; 105:1791–1799. PMID: 32826797.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multivariable linear model for predicting graft weight based on three-dimensional volumetry in regards of body weight change of living liver donor
- The correlation between preoperative volumetry and real graft weight: comparison of two volumetry programs
- Liver Regeneration and Factors Influencing Liver Regeneration in Donors and Recipients of Adult Living Donor Liver Transplantation Using Right Lobe Graft
- Clinical implication of hepatic volumetry for living donor liver transplantation
- Impact of Graft Kidney Volume and Weight on Graft Function in Living Donor Kidney Transplantation