Korean J Orthod.
2024 Jul;54(4):199-209. 10.4041/kjod24.085.
Intraoral ageing of aligners and attachments: Adverse effects on clinical efficiency and release of biologically-active compounds
- Affiliations
-
- 1Clinic of Orthodontics and Pediatric Dentistry, Center for Dental Medicine, University of Zurich, Zurich, Switzerland
- 2Department of Biomaterials, School of Dentistry, National and Kapodistrian University of Athens, Athens, Greece
- KMID: 2558290
- DOI: http://doi.org/10.4041/kjod24.085
Abstract
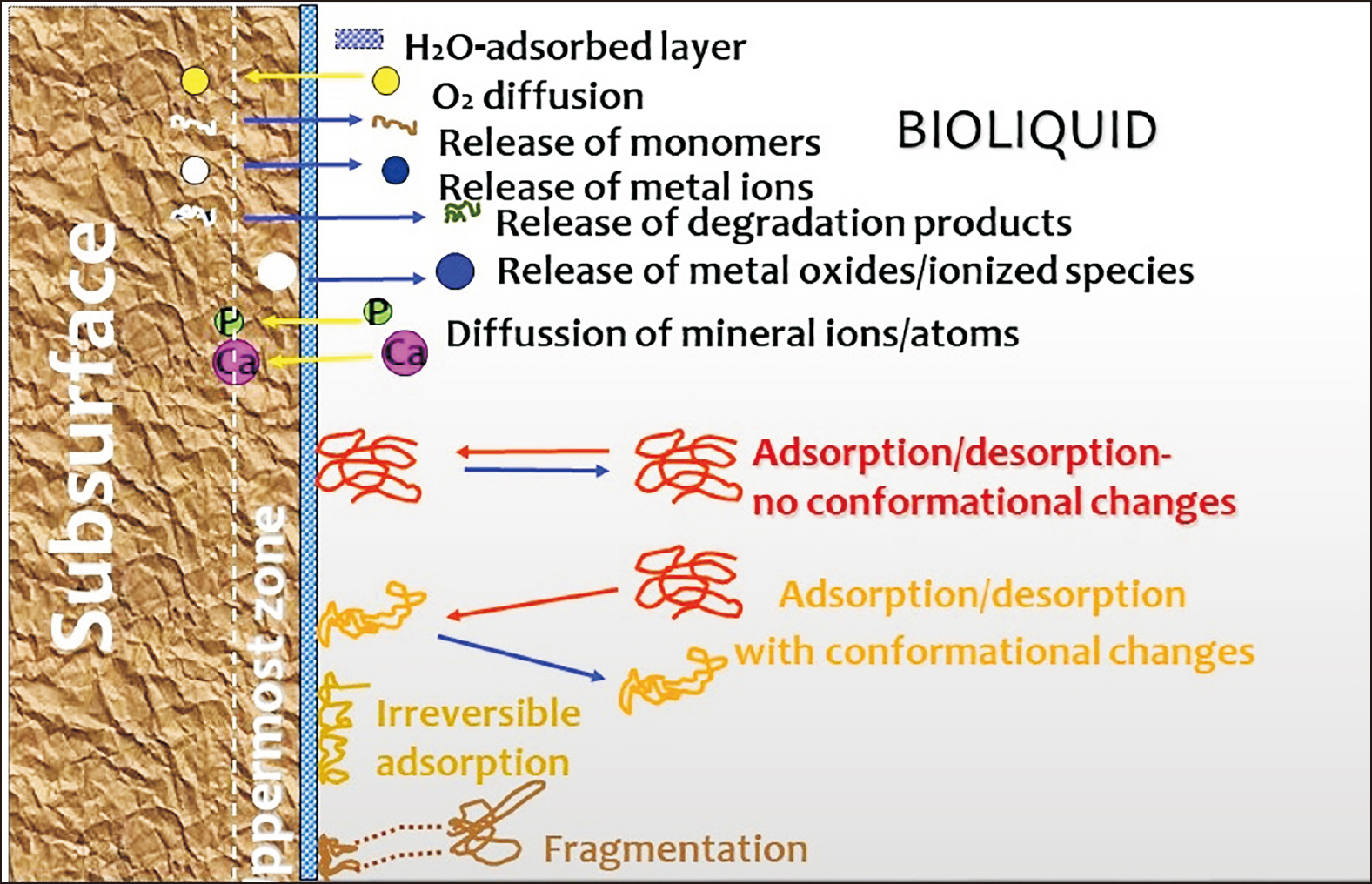
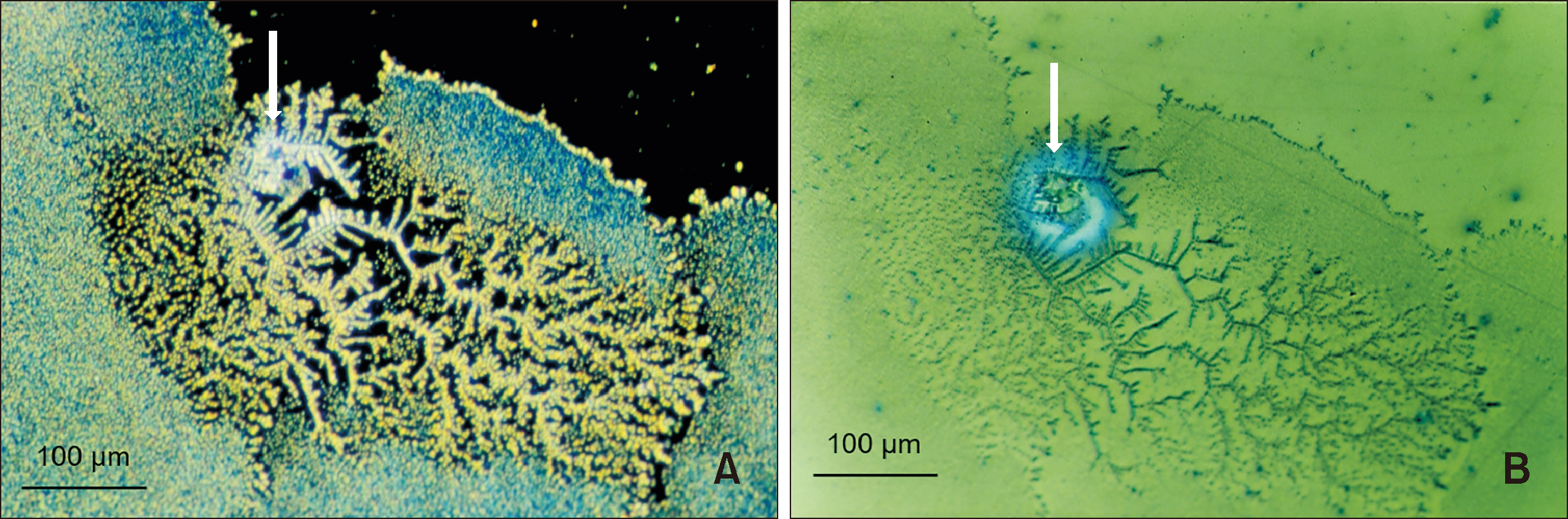
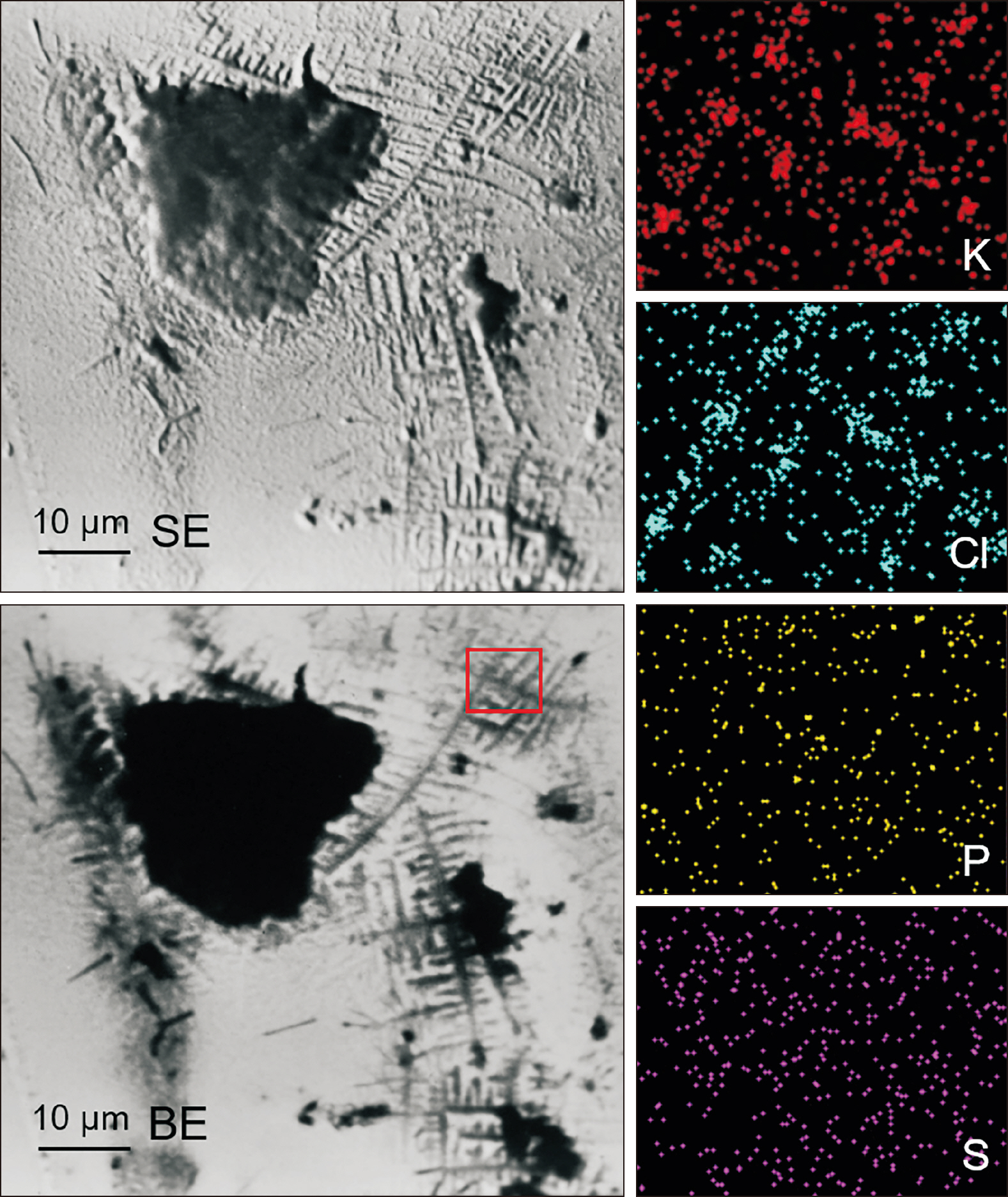
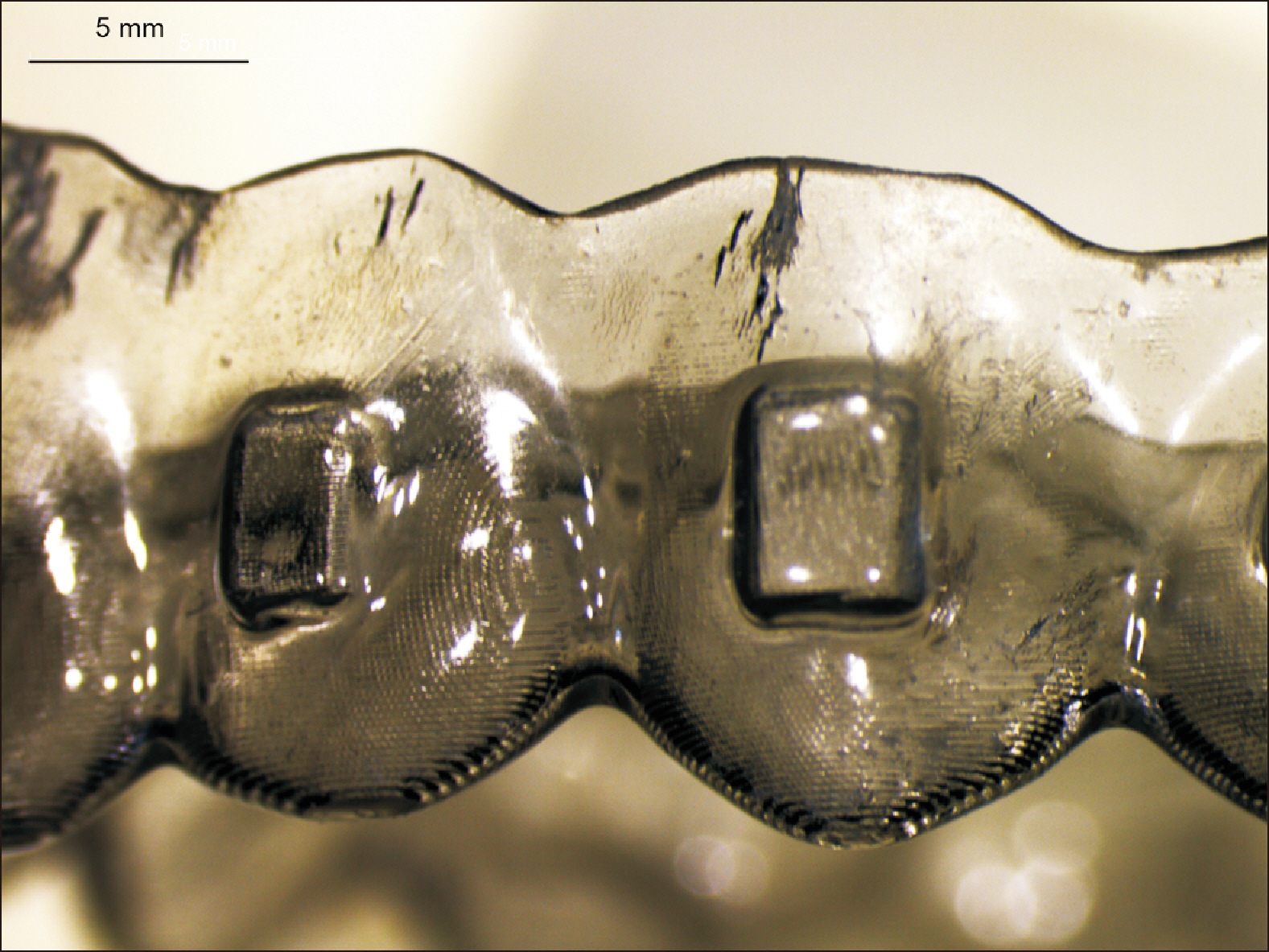
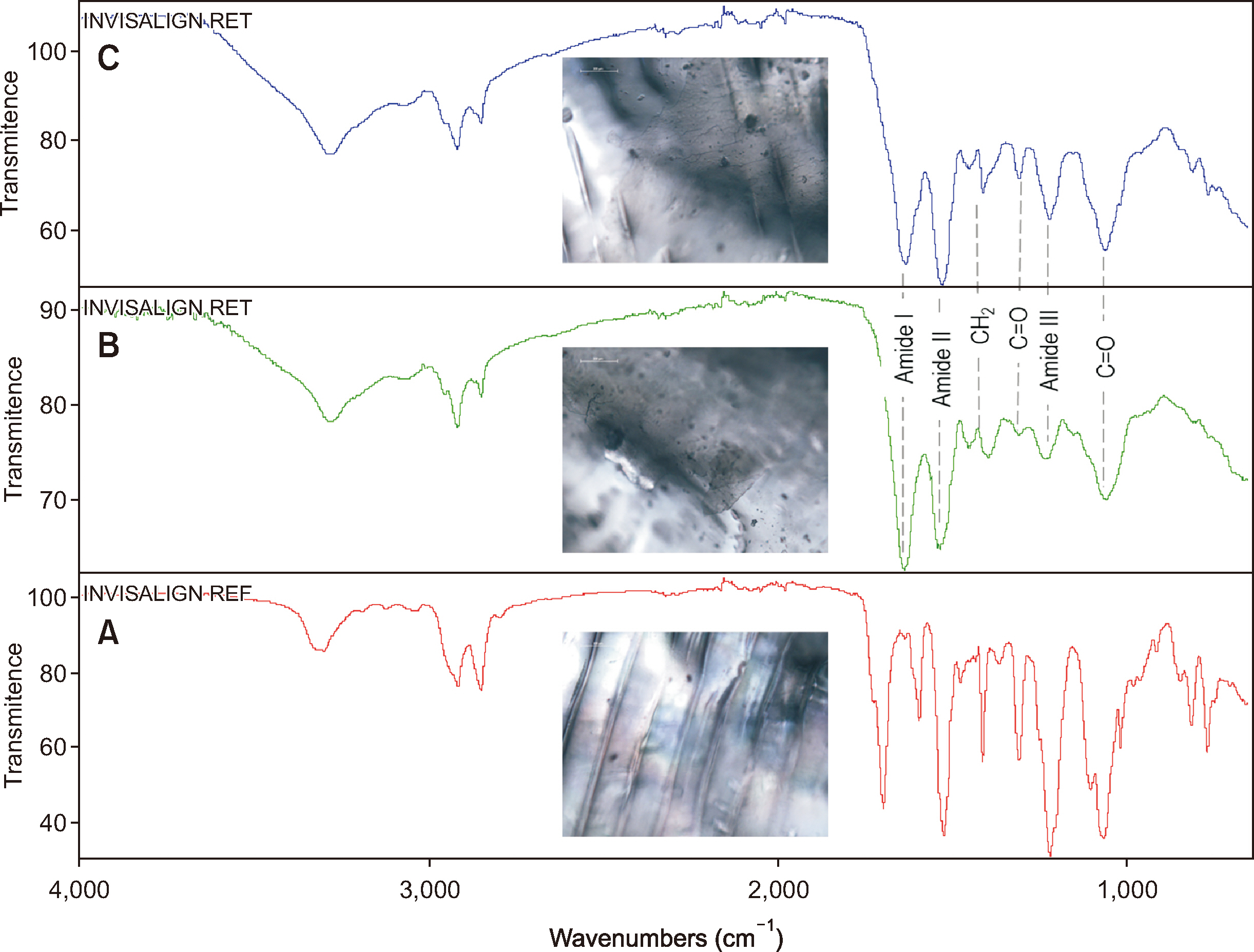
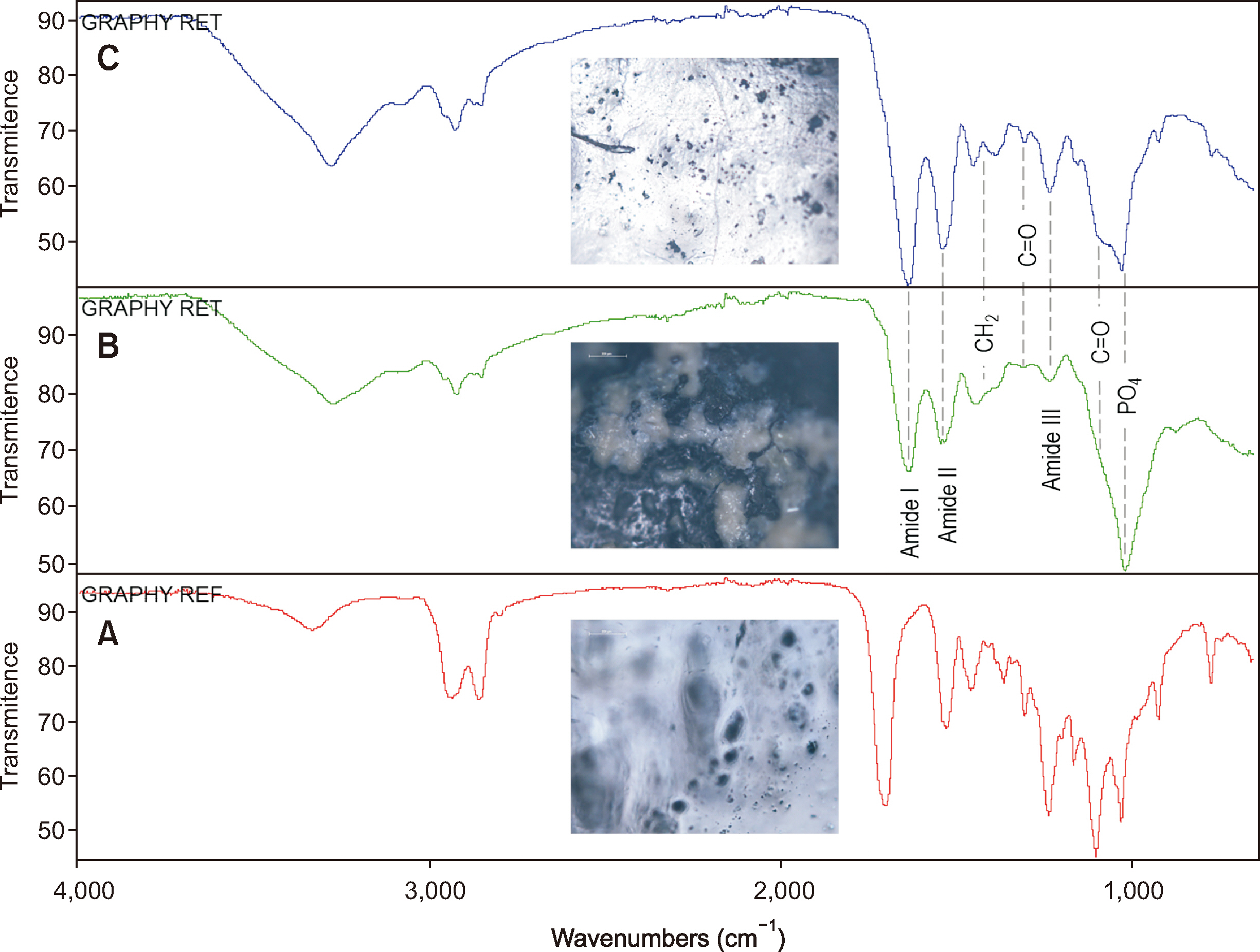
- The clinical application of aligners is accompanied by the ageing of the polymer appliances and the attachments used, which may result in inefficiency in reaching the predicted range of tooth movement, and release of compounds and microplastics in the oral cavity as a result of the friction, wear and attrition of the aligner and composite attachment. The purpose of this review is to present the mechanism and effects of in vivo ageing; describe the hydrolytic degradation of aligners and enzymatic degradation of composite attachments; examine the ageing pattern of aligners in vivo, under actual clinical scenarios; and identify a link to the discrepancy between predicted and actual clinical outcome. Lastly, strategies to deal with three potentially critical issues associated with the use of aligners, namely the necessity of weekly renewal, the dissimilar mechanical properties of aligner and attachment resulting in wear and plastic deformation of the aligner, and the development of integuments and biofilms with microbial colonization of the appliance, are discussed.
Keyword
Figure
Reference
-
References
1. Papageorgiou SN, Koletsi D, Iliadi A, Peltomaki T, Eliades T. 2020; Treatment outcome with orthodontic aligners and fixed appliances: a systematic review with meta-analyses. Eur J Orthod. 42:331–43. https://doi.org/10.1093/ejo/cjz094. DOI: 10.1093/ejo/cjz094. PMID: 31758191.
Article2. Quirynen M, Marechal M, Busscher HJ, Weerkamp AH, Arends J, Darius PL, et al. 1989; The influence of surface free-energy on planimetric plaque growth in man. J Dent Res. 68:796–9. https://doi.org/10.1177/00220345890680050801. DOI: 10.1177/00220345890680050801. PMID: 2715472.
Article3. Merrill EW. 1987; Distinctions and correspondences among surfaces contacting blood. Ann N Y Acad Sci. 516:196–203. https://doi.org/10.1111/j.1749-6632.1987.tb33041.x. DOI: 10.1111/j.1749-6632.1987.tb33041.x. PMID: 3439726.
Article4. Karino T, Goldsmith HL, Motomiya M, Mabuchi S, Sohara Y. 1987; Flow patterns in vessels of simple and complex geometries. Ann N Y Acad Sci. 516:422–41. https://doi.org/10.1111/j.1749-6632.1987.tb33063.x. DOI: 10.1111/j.1749-6632.1987.tb33063.x. PMID: 3439740.
Article5. Kasemo B, Lausmaa J. Davies JE, editor. 1991. The biomaterial-tissue interface and its analogues in surface science and technology. Bone-bio material interface. University of Toronto Press;Toronto: p. 19–32. https://doi.org/10.3138/9781442671508-005. DOI: 10.3138/9781442671508-005. PMID: 1960574.
Article6. Eliades G, Eliades T, Vavuranakis M. Eliades G, Eliades T, Brantley WA, Watts DC, editors. 2003. General aspects of biomaterial surface alterations following exposure to biologic fluids. Dental materials in vivo: aging and related phenomena. Quintessence Publishing Co.;Chicago: p. 3–22. https://www.quintessence-publishing.com/gbr/en/product/dental-materials-in-vivo-aging-and-related-phenomena. DOI: 10.1016/s0889-5406(03)00538-9.7. Israelachvili J. 2011. Intermolecular and surface forces. 3rd ed. Academic Press;San Diego: p. 275–86. https://doi.org/10.1016/C2009-0-21560-1. DOI: 10.1016/C2009-0-21560-1.
Article8. Brash JL. Missirlis YF, Lemm W, editors. 1991. Studies of protein adsorption relevant to blood compatible materials. Modern aspects of protein adsorption on biomaterials. Kluwer Academic Press;Dordrecht: p. 39–47. https://doi.org/10.1007/978-94-011-3752-2_5. DOI: 10.1007/978-94-011-3752-2_5.
Article9. Andrade JD, Hlady V. 1987; Plasma protein adsorption: the big twelve. Ann N Y Acad Sci. 516:158–72. https://doi.org/10.1111/j.1749-6632.1987.tb33038.x. DOI: 10.1111/j.1749-6632.1987.tb33038.x. PMID: 3439723.
Article10. Wood SR, Kirkham J, Marsh PD, Shore RC, Nattress B, Robinson C. 2000; Architecture of intact natural human plaque biofilms studied by confocal laser scanning microscopy. J Dent Res. 79:21–7. https://doi.org/10.1177/00220345000790010201. DOI: 10.1177/00220345000790010201. PMID: 10690656.
Article11. Zinelis S, Polychronis G, Papadopoulos F, Kokkinos C, Economou A, Panayi N, et al. 2022; Mechanical and electrochemical characterization of 3D printed orthodontic metallic appliances after in vivo ageing. Dent Mater. 38:1721–7. https://doi.org/10.1016/j.dental.2022.09.002. DOI: 10.1016/j.dental.2022.09.002. PMID: 36123188.
Article12. Papadopoulou AK, Cantele A, Polychronis G, Zinelis S, Eliades T. 2019; Changes in roughness and mechanical properties of Invisalign® appliances after one- and two-weeks use. Materials (Basel). 12:2406. https://doi.org/10.3390/ma12152406. DOI: 10.3390/ma12152406. PMID: 31357697. PMCID: PMC6696190. PMID: db94c46c52ee400fa864a2d4fd2053a5.
Article13. Schätzle M, Zinelis S, Markic G, Eliades G, Eliades T. 2017; Structural, morphological, compositional, and mechanical changes of palatal implants after use: a retrieval analysis. Eur J Orthod. 39:579–85. https://doi.org/10.1093/ejo/cjx001. DOI: 10.1093/ejo/cjx001. PMID: 28199512.14. Gugger J, Pandis N, Zinelis S, Patcas R, Eliades G, Eliades T. 2016; Retrieval analysis of lingual fixed retainer adhesives. Am J Orthod Dentofacial Orthop. 150:575–84. https://doi.org/10.1016/j.ajodo.2016.06.012. DOI: 10.1016/j.ajodo.2016.06.012. PMID: 27692414.
Article15. Hersche S, Sifakakis I, Zinelis S, Eliades T. 2017; Elemental, microstructural, and mechanical characterization of high gold orthodontic brackets after intraoral aging. Biomed Tech (Berl). 62:97–102. https://doi.org/10.1515/bmt-2015-0205. DOI: 10.1515/bmt-2015-0205. PMID: 27049606.
Article16. Soteriou D, Ntasi A, Papagiannoulis L, Eliades T, Zinelis S. 2014; Decomposition of Ag-based soldering alloys used in space maintainers after intra-oral exposure. A retrieval analysis study. Acta Odontol Scand. 72:130–8. https://doi.org/10.3109/00016357.2013.812745. DOI: 10.3109/00016357.2013.812745. PMID: 23822908.
Article17. Eliades T, Zinelis S, Papadopoulos MA, Eliades G. 2009; Characterization of retrieved orthodontic miniscrew implants. Am J Orthod Dentofacial Orthop. 135:10.e1–7. discussion 10–1. https://doi.org/10.1016/j.ajodo.2008.06.019. DOI: 10.1016/j.ajodo.2008.06.019. PMID: 19121491.18. Zinelis S, Eliades T, Dimitrakopoulos I, Silikas N, Eliades G. 2009; Surface characterization and force relaxation of retrieved silk sutures. J Biomed Mater Res B Appl Biomater. 89:551–7. https://doi.org/10.1002/jbm.b.31248. DOI: 10.1002/jbm.b.31248. PMID: 18985772.
Article19. Schuster S, Eliades G, Zinelis S, Eliades T, Bradley TG. 2004; Structural conformation and leaching from in vitro aged and retrieved Invisalign appliances. Am J Orthod Dentofacial Orthop. 126:725–8. https://doi.org/10.1016/j.ajodo.2004.04.021. DOI: 10.1016/j.ajodo.2004.04.021. PMID: 15592222.20. Eliades T, Papadopulos JS, Eliades G, Silikas N, Watts DC. 2003; Multi-technique characterization of retrieved bone cement from revised total hip arthroplasties. J Mater Sci Mater Med. 14:967–72. https://doi.org/10.1023/a:1026350616079. DOI: 10.1023/A:1026350616079. PMID: 15348509.21. Eliades T, Athanasiou AE. 2002; In vivo aging of orthodontic alloys: implications for corrosion potential, nickel release, and biocompatibility. Angle Orthod. 72:222–37. https://pubmed.ncbi.nlm.nih.gov/12071606/.22. Eliades T, Eliades G, Watts DC. 2001; Intraoral aging of the inner headgear component: a potential biocompatibility concern? Am J Orthod Dentofacial Orthop. 119:300–6. https://doi.org/10.1067/mod.2001.111402. DOI: 10.1067/mod.2001.111402. PMID: 11244424.
Article23. Eliades T, Eliades G, Athanasiou AE, Bradley TG. 2000; Surface characterization of retrieved NiTi orthodontic archwires. Eur J Orthod. 22:317–26. https://doi.org/10.1093/ejo/22.3.317. DOI: 10.1093/ejo/22.3.317. PMID: 10920564.
Article24. Eliades T, Eliades G, Watts DC. 1999; Structural conformation of in vitro and in vivo aged orthodontic elastomeric modules. Eur J Orthod. 21:649–58. https://doi.org/10.1093/ejo/21.6.649. DOI: 10.1093/ejo/21.6.649. PMID: 10665194.
Article25. Magnissalis EA, Eliades G, Eliades T. 1999; Multitechnique characterization of articular surfaces of retrieved ultrahigh molecular weight polyethylene acetabular sockets. J Biomed Mater Res. 48:365–73. https://doi.org/10.1002/(sici)1097-4636(1999)48:3<365::aid-jbm22>3.0.co;2-t. DOI: 10.1002/(SICI)1097-4636(1999)48:3<365::AID-JBM22>3.0.CO;2-T.
Article26. Vassilakos N. 1992. Some biophysical aspects of salivary film formation. Studies of salivary adsorption at solid/liquid and air/liquid interfaces [PhD dissertation]. Lund University;Lund: DOI: 10.1016/0003-9969(92)90137-W. PMID: 1332664.27. Embery G, Hogg SD, Heaney TG, Stanbuty JB, Green RDJ. ten Cate JM, Leach SA, Arends J, editors. 1984. Some considerations on dental pellicle formation and early bacterial colonization: the role of high and low molecular weight proteins of the major and minor salivary glands. Bacterial adhesion and preventive dentistry. IRL Press;Oxford: p. 73–84.28. Busscher HJ, van der Mei HC. 1997; Physico-chemical interactions in initial microbial adhesion and relevance for biofilm formation. Adv Dent Res. 11:24–32. https://doi.org/10.1177/08959374970110011301. DOI: 10.1177/08959374970110011301. PMID: 9524439.
Article29. Christersson CE, Dunford RG, Glantz PO, Baier RE. 1989; Effect of critical surface tension on retention of oral microorganisms. Scand J Dent Res. 97:247–56. https://doi.org/10.1111/j.1600-0722.1989.tb01609.x. DOI: 10.1111/j.1600-0722.1989.tb01609.x. PMID: 2740836.
Article30. Bowden GH, Li YH. 1997; Nutritional influences on biofilm development. Adv Dent Res. 11:81–99. https://doi.org/10.1177/08959374970110012101. DOI: 10.1177/08959374970110012101. PMID: 9524446.31. Vasin SL, Rosanova IB, Sevastianov VI. 1998; The role of proteins in the nucleation and formation of calcium-containing deposits on biomaterial surfaces. J Biomed Mater Res. 39:491–7. https://doi.org/10.1002/(sici)1097-4636(19980305)39:3<491::aid-jbm21>3.0.co;2-c. DOI: 10.1002/(SICI)1097-4636(19980305)39:3<491::AID-JBM21>3.0.CO;2-C. PMID: 9468061.
Article32. Quirynen M, Marechal M, Busscher HJ, Weerkamp AH, Darius PL, van Steenberghe D. 1990; The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J Clin Periodontol. 17:138–44. https://doi.org/10.1111/j.1600-051x.1990.tb01077.x. DOI: 10.1111/j.1600-051X.1990.tb01077.x. PMID: 2319000.
Article33. Yamauchi M, Yamamoto K, Wakabayashi M, Kawano J. 1990; In vitro adherence of microorganisms to denture base resin with different surface texture. Dent Mater J. 9:19–24. https://doi.org/10.4012/dmj.9.19. DOI: 10.4012/dmj.9.19. PMID: 2098207.34. Siegrist BE, Brecx MC, Gusberti FA, Joss A, Lang NP. 1991; In vivo early human dental plaque formation on different supporting substances. A scanning electron microscopic and bacteriological study. Clin Oral Implants Res. 2:38–46. https://doi.org/10.1034/j.1600-0501.1991.020105.x. DOI: 10.1034/j.1600-0501.1991.020105.x. PMID: 1807421.
Article35. Hannig M. 1999; Transmission electron microscopy of early plaque formation on dental materials in vivo. Eur J Oral Sci. 107:55–64. https://doi.org/10.1046/j.0909-8836.1999.eos107109.x. DOI: 10.1046/j.0909-8836.1999.eos107109.x. PMID: 10102751.
Article36. Yao Y, Grogan J, Zehnder M, Lendenmann U, Nam B, Wu Z, et al. 2001; Compositional analysis of human acquired enamel pellicle by mass spectrometry. Arch Oral Biol. 46:293–303. https://doi.org/10.1016/s0003-9969(00)00134-5. DOI: 10.1016/S0003-9969(00)00134-5. PMID: 11269863.
Article37. Possart W, Zimmer B. 2024; Water in polyurethane networks: physical and chemical ageing effects and mechanical parameters. Contin Mech Thermodyn. 36:261–87. https://doi.org/10.1007/s00161-022-01082-y. DOI: 10.1007/s00161-022-01082-y.
Article38. Kozakiewicz J, Rokicki G, Przybylski J, Sylwestrzak K, Parzuchowski PG, Tomczyk KM. 2010; Studies of the hydrolytic stability of poly(urethane-urea) elastomers synthesized from oligocarbonate diols. Polym Degrad Stab. 95:2413–20. https://doi.org/10.1016/j.polymdegradstab.2010.08.017. DOI: 10.1016/j.polymdegradstab.2010.08.017.39. Arhant M, Le Gall M, Le Gac PY, Davies P. 2019; Impact of hydrolytic degradation on mechanical properties of PET- towards an understanding of microplastics formation. Polym Degrad Stab. 161:175–82. https://doi.org/10.1016/j.polymdegradstab.2019.01.021. DOI: 10.1016/j.polymdegradstab.2019.01.021.
Article40. Shokati B, Tam LE, Santerre JP, Finer Y. 2010; Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface. J Biomed Mater Res B Appl Biomater. 94:230–7. https://doi.org/10.1002/jbm.b.31645. DOI: 10.1002/jbm.b.31645. PMID: 20524199.
Article41. Finer Y, Jaffer F, Santerre JP. 2004; Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites. Biomaterials. 25:1787–93. https://doi.org/10.1016/j.biomaterials.2003.08.029. DOI: 10.1016/j.biomaterials.2003.08.029. PMID: 14738842.
Article42. Marashdeh MQ, Gitalis R, Levesque C, Finer Y. 2018; Enterococcus faecalis hydrolyzes dental resin composites and adhesives. J Endod. 44:609–13. https://doi.org/10.1016/j.joen.2017.12.014. DOI: 10.1016/j.joen.2017.12.014. PMID: 29397213. PMCID: PMC5869143.
Article43. Bourbia M, Ma D, Cvitkovitch DG, Santerre JP, Finer Y. 2013; Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res. 92:989–94. https://doi.org/10.1177/0022034513504436. DOI: 10.1177/0022034513504436. PMID: 24026951. PMCID: PMC3797536.
Article44. Nedeljkovic I, De Munck J, Ungureanu AA, Slomka V, Bartic C, Vananroye A, et al. 2017; Biofilm-induced changes to the composite surface. J Dent. 63:36–43. https://doi.org/10.1016/j.jdent.2017.05.015. DOI: 10.1016/j.jdent.2017.05.015. PMID: 28554609.
Article45. Rios-Madrigal AM, Orea-Vega DC, Vega-González M, Espinosa-Cristóbal LF, Arenas-Arrocena MC, Castro-Ruiz JE, et al. 2021; Effect of Streptococcus mutans on surface-topography, microhardness, and mechanical properties of contemporary resin composites. J Appl Biomater Funct Mater. 19:22808000211065260. https://doi.org/10.1177/22808000211065260. DOI: 10.1177/22808000211065260. PMID: 34915756. PMID: 495821ad3ad346579b3fead6e9c57606.46. Gitalis R, Zhou L, Marashdeh MQ, Sun C, Glogauer M, Finer Y. 2019; Human neutrophils degrade methacrylate resin composites and tooth dentin. Acta Biomater. 88:325–31. https://doi.org/10.1016/j.actbio.2019.02.033. DOI: 10.1016/j.actbio.2019.02.033. PMID: 30807874. PMCID: PMC6491217.47. Ahmadieh S, Kim HW, Weintraub NL. 2020; Potential role of perivascular adipose tissue in modulating atherosclerosis. Clin Sci (Lond). 134:3–13. https://doi.org/10.1042/CS20190577. DOI: 10.1042/CS20190577. PMID: 31898749. PMCID: PMC6944729.
Article48. Zhu X, Wang C, Duan X, Liang B, Genbo Xu E, Huang Z. 2023; Micro- and nanoplastics: a new cardiovascular risk factor? Environ Int. 171:107662. https://doi.org/10.1016/j.envint.2022.107662. DOI: 10.1016/j.envint.2022.107662. PMID: 36473237.
Article49. Marfella R, Prattichizzo F, Sardu C, Fulgenzi G, Graciotti L, Spadoni T, et al. 2024; Microplastics and nanoplastics in atheromas and cardiovascular events. N Engl J Med. 390:900–10. https://doi.org/10.1056/NEJMoa2309822. DOI: 10.1056/NEJMoa2309822. PMID: 38446676.
Article50. Quinzi V, Orilisi G, Vitiello F, Notarstefano V, Marzo G, Orsini G. 2023; A spectroscopic study on orthodontic aligners: first evidence of secondary microplastic detachment after seven days of artificial saliva exposure. Sci Total Environ. 866:161356. https://doi.org/10.1016/j.scitotenv.2022.161356. DOI: 10.1016/j.scitotenv.2022.161356. PMID: 36603638.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Force and moment analysis of clear aligners: Impact of material properties and design on premolar rotation
- Evaluation of the effect of attachments on torque control of palatally positioned maxillary lateral teeth with clear aligners: Finite element analysis
- Comparison of the predicted and achieved labiolingual inclinations of the maxillary central incisors in adult Class II division 2 malocclusions treated with clear aligners
- Psychosocial Aspects of Normal Ageing
- Evaluation of Two Biologically Active Compounds for Control of Wheat Root Rot and its Causal Pathogens