Intest Res.
2024 Jul;22(3):378-386. 10.5217/ir.2023.00203.
Live-attenuated vaccination in patients with inflammatory bowel disease while continuing or after elective switch to vedolizumab
- Affiliations
-
- 1Division of Gastroenterology, Tohoku University Graduate School of Medicine, Sendai, Japan
- 2Student Health Care Center, Institute for Excellence in Higher Education, Tohoku University, Sendai, Japan
- KMID: 2558197
- DOI: http://doi.org/10.5217/ir.2023.00203
Abstract
- Background/Aims
Vedolizumab (VDZ) is a gut-selective agent with a favorable safety profile. We aimed to assess the feasibility of elective switch from other advanced therapies to VDZ and subsequent live-attenuated vaccination while continuing VDZ in patients with inflammatory bowel diseases (IBD).
Methods
We measured antibody titers specific for measles, rubella, mumps, and varicella viruses in IBD patients under immunosuppressive therapy. Those with negative titers and without vaccination history were judged unimmunized. Patients were administered vaccines while continuing VDZ or switched to VDZ if receiving other advanced therapies and then administered vaccines. Co-primary outcomes were the rate of maintaining disease severity after vaccination and the rate without vaccine-induced infection.
Results
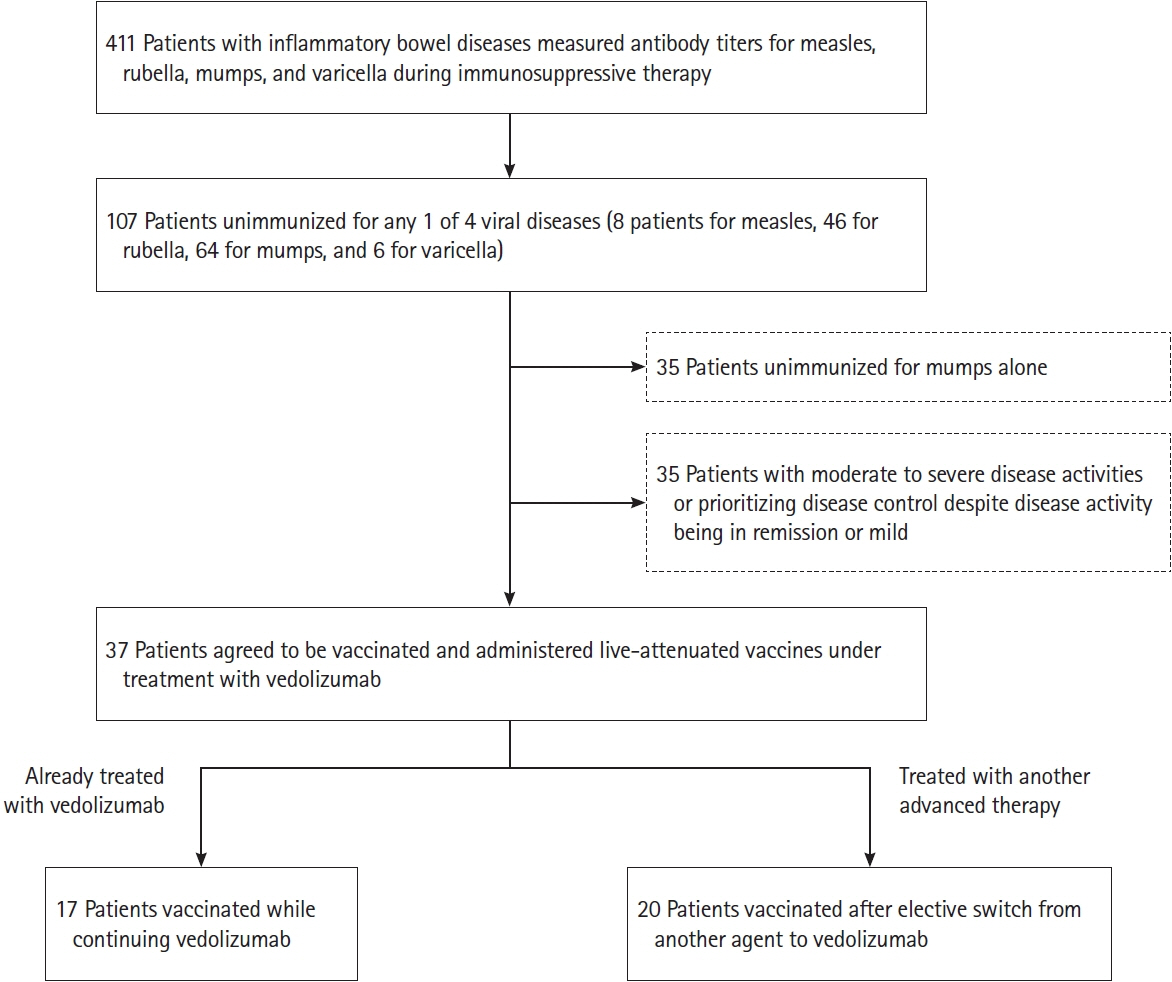
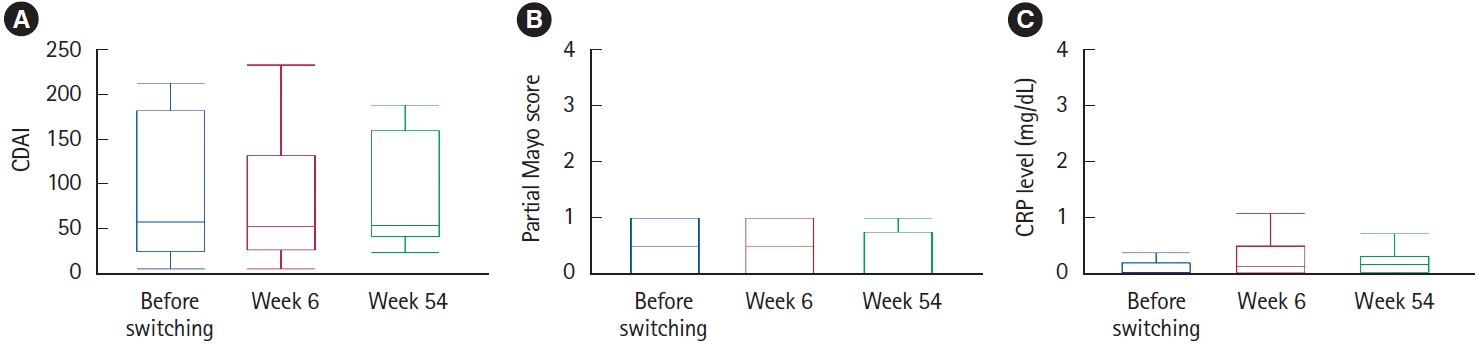
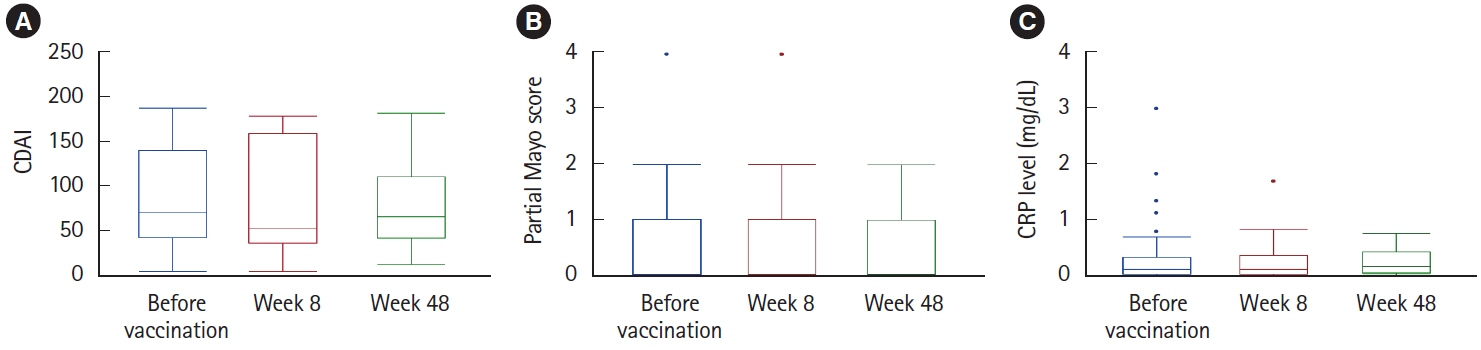
Among 107 unimmunized patients, 37 agreed to receive live-attenuated vaccines while continuing VDZ (17 patients) or after switching to VDZ (20 patients). In the 20 patients who electively switched to VDZ, disease severity was maintained except for 1 patient who developed intestinal infection. After 54 weeks, 18 patients (90%) continued to receive VDZ, excluding 2 patients who reverted to their originally administered biologics. In all 37 patients administered live-attenuated vaccines under VDZ treatment, disease severity was maintained after vaccination. Antibody titers became positive or equivocal in 34 patients (91.9%). There were no cases of vaccine-induced infection during a median observation period of 121 weeks.
Conclusions
While live-attenuated vaccines are contraindicated under immunosuppressive therapy, they may be safely administered while receiving VDZ immunotherapy. Switching from other advanced therapies to VDZ and subsequently receiving live-attenuated vaccines may be a safe alternative in unimmunized patients.
Figure
Reference
-
1. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017; 66:839–851.
Article2. Ng SC, Hilmi IN, Blake A, et al. Low frequency of opportunistic infections in patients receiving vedolizumab in clinical trials and post-marketing setting. Inflamm Bowel Dis. 2018; 24:2431–2441.
Article3. Meserve J, Aniwan S, Koliani-Pace JL, et al. Retrospective analysis of safety of vedolizumab in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019; 17:1533–1540.
Article4. Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. Firstand second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020; 18:2179–2191.5. Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022; 7:161–170.
Article6. Wang Y, Wang J, Pekow J, et al. Outcome of elective switching to vedolizumab in inflammatory bowel disease patients under tumor necrosis factor antagonist-maintained clinical remission. J Gastroenterol Hepatol. 2019; 34:2090–2095.
Article7. Naganuma M, Nagahori M, Fujii T, Morio J, Saito E, Watanabe M. Poor recall of prior exposure to varicella zoster, rubella, measles, or mumps in patients with IBD. Inflamm Bowel Dis. 2013; 19:418–422.
Article8. Shiga H, Takahashi T, Shiraki M, et al. Reduced antiviral seropositivity among patients with inflammatory bowel disease treated with immunosuppressive agents. Scand J Gastroenterol. 2023; 58:360–367.
Article9. Lambert N, Strebel P, Orenstein W, Icenogle J, Poland GA. Rubella. Lancet. 2015; 385:2297–2307.
Article10. Saitoh A, Okabe N. Progress and challenges for the Japanese immunization program: beyond the “vaccine gap”. Vaccine. 2018; 36:4582–4588.
Article11. Phadke VK, Bednarczyk RA, Salmon DA, Omer SB. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. JAMA. 2016; 315:1149–1158.
Article12. Cohen ER, Salem M, Ha C. Managing immunosuppressed patients with inflammatory bowel disease during a measles outbreak. Am J Gastroenterol. 2019; 114:1563–1565.
Article13. Feldman AG, O’Leary ST, Danziger-Isakov L. The risk of resurgence in vaccine-preventable infections due to coronavirus disease 2019-related gaps in immunization. Clin Infect Dis. 2021; 73:1920–1923.
Article14. Hübschen JM, Gouandjika-Vasilache I, Dina J. Measles. Lancet. 2022; 399:678–690.
Article15. Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021; 15:879–913.
Article16. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019; 68(Suppl 3):s1–s106.
Article17. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG Clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017; 112:241–258.
Article18. Gisbert JP, Chaparro M. Vaccination strategies in patients with IBD. Nat Rev Gastroenterol Hepatol. 2013; 10:277–285.
Article19. Benchimol EI, Tse F, Carroll MW, et al. Canadian Association of Gastroenterology clinical practice guideline for immunizations in patients with inflammatory bowel disease (IBD) part 1. Live vaccines. Gastroenterology. 2021; 161:669–680.
Article20. Ishige T, Shimizu T, Watanabe K, et al. Expert consensus on vaccination in patients with inflammatory bowel disease in Japan. J Gastroenterol. 2023; 58:135–157.
Article21. Wichmann A, Krugliak Cleveland N, Rubin DT. Safety and efficacy of live measles vaccine administered to a Crohn’s disease patient receiving vedolizumab. Am J Gastroenterol. 2016; 111:577.
Article22. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article23. Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021; 56:489–526.
Article24. Caldera F, Misch EA, Saha S, et al. Immunosuppression does not affect antibody concentrations to measles, mumps, and rubella in patients with inflammatory bowel disease. Dig Dis Sci. 2019; 64:189–195.
Article25. Croce E, Hatz C, Jonker EF, Visser LG, Jaeger VK, Bühler S. Safety of live vaccinations on immunosuppressive therapy in patients with immune-mediated inflammatory diseases, solid organ transplantation or after bone-marrow transplantation: a systematic review of randomized trials, observational studies and case reports. Vaccine. 2017; 35:1216–1226.
Article26. Manser CN, Maillard MH, Rogler G, et al. Vaccination in patients with inflammatory bowel diseases. Digestion. 2020; 101 Suppl 1:58–68.
Article27. Alexander JL, Kennedy NA, Ibraheim H, et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol. 2022; 7:342–352.
Article28. Shiga H, Kakuta Y, An K, et al. Response to COVID-19 vaccine is reduced in patients with inflammatory bowel disease, but improved with additional dose. J Gastroenterol Hepatol. 2023; 38:44–51.29. Liu Z, Alexander JL, Yee Eng K, et al. Antibody responses to Influenza vaccination are diminished in patients with inflammatory bowel disease on infliximab or tofacitinib. J Crohns Colitis. 2024; 18:560–569.
Article30. Cleveland NK, Rodriquez D, Wichman A, Pan I, Melmed GY, Rubin DT. Many inflammatory bowel disease patients are not immune to measles or pertussis. Dig Dis Sci. 2016; 61:2972–2976.
Article31. deBruyn JC, Soon IS, Fonseca K, et al. Serologic status of routine childhood vaccines, cytomegalovirus, and Epstein-Barr virus in children with inflammatory bowel disease. Inflamm Bowel Dis. 2019; 25:1218–1226.
Article32. Khan N, Trivedi C, Aberra F, Pernes T, Yang YX. Safety of recombinant zoster vaccine in patients with inflammatory bowel disease. J Crohns Colitis. 2022; 16:1505–1507.
Article33. Khan N, Wang L, Trivedi C, et al. Efficacy of recombinant zoster vaccine in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2022; 20:1570–1578.
Article34. Cullen G, Baden RP, Cheifetz AS. Varicella zoster virus infection in inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:2392–2403.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vaccination in Inflammatory Rheumatic Diseases
- Herpes zoster infection in patients with inflammatory bowel disease
- The Risk of Tuberculosis in Patients With Inflammatory Bowel Disease Treated With Vedolizumab or Ustekinumab in Korea
- Treatment of inflammatory bowel diseases: focusing on biologic agents and new therapies
- Old and New Biologics and Small Molecules in Inflammatory Bowel Disease: Anti Integrins