Intest Res.
2024 Jul;22(3):297-309. 10.5217/ir.2023.00129.
Intestinal ultrasound for intestinal Behçet disease reflects endoscopic activity and histopathological findings
- Affiliations
-
- 1Inflammatory Bowel Disease Center, Yokohama City University Medical Center, Yokohama, Japan
- 2Department of Gastroenterology, Yokohama City University, Graduate School of Medicine, Yokohama, Japan
- 3Department of Laboratory Medicine and Clinical Investigation, Yokohama City University Medical Center, Yokohama, Japan
- 4Department of Diagnostic Pathology, Yokohama City University Medical Center, Yokohama, Japan
- 5Department of Molecular Pathology, Yokohama City University, Graduate School of Medicine, Yokohama, Japan
- 6Gastroenterological Center, Yokohama City University Medical Center, Yokohama, Japan
- KMID: 2558190
- DOI: http://doi.org/10.5217/ir.2023.00129
Abstract
- Background/Aims
Intestinal Behçet disease is typically associated with ileocecal punched-out ulcers and significant morbidity and mortality. Intestinal ultrasound is a noninvasive imaging technique for disease monitoring. However, no previous reports have compared intestinal ultrasound with endoscopic ulcer activity or histopathological findings for intestinal Behçet disease. We evaluated the usefulness of intestinal ultrasound for assessing the activity of ileocecal ulcers in intestinal Behçet disease.
Methods
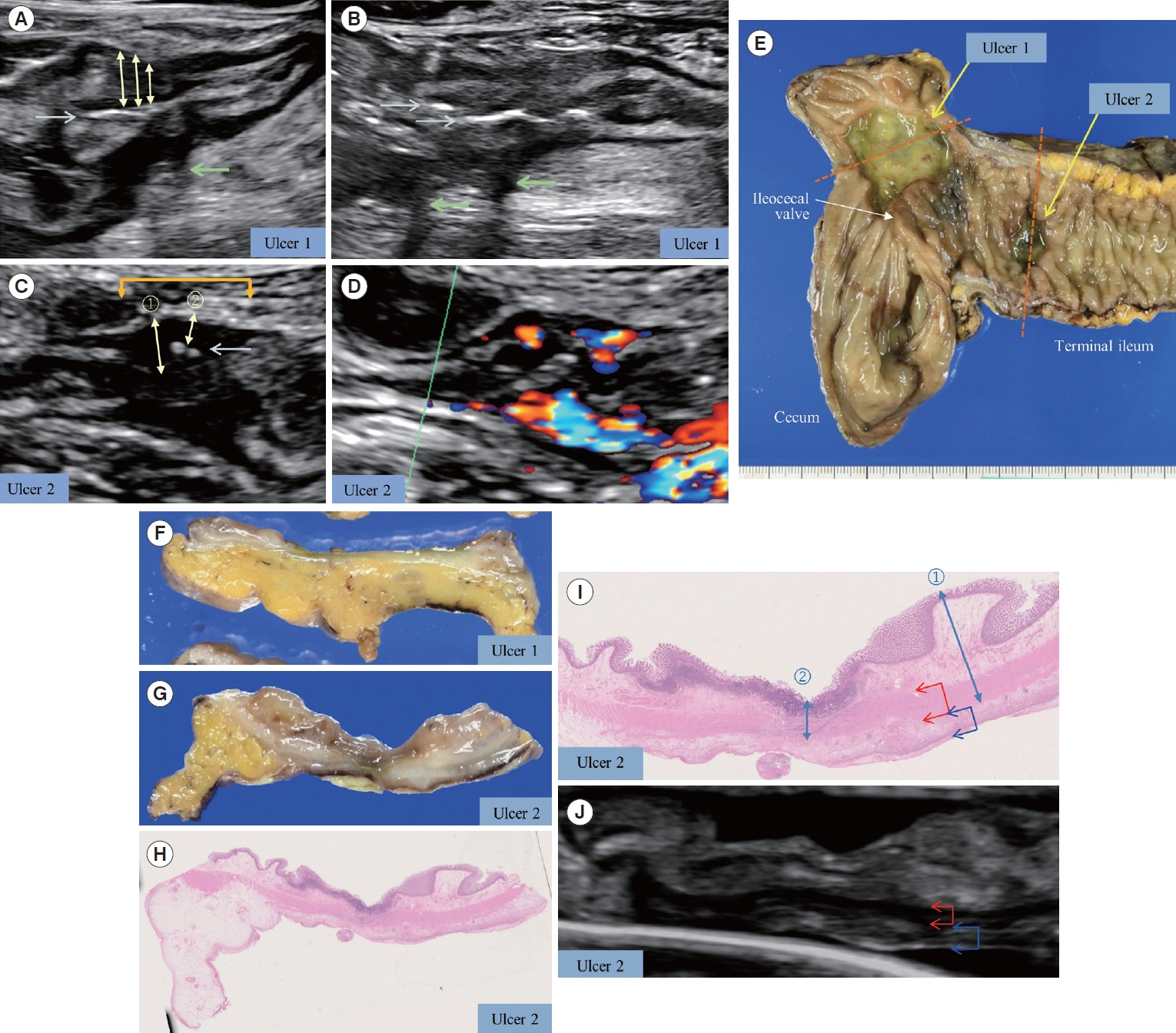
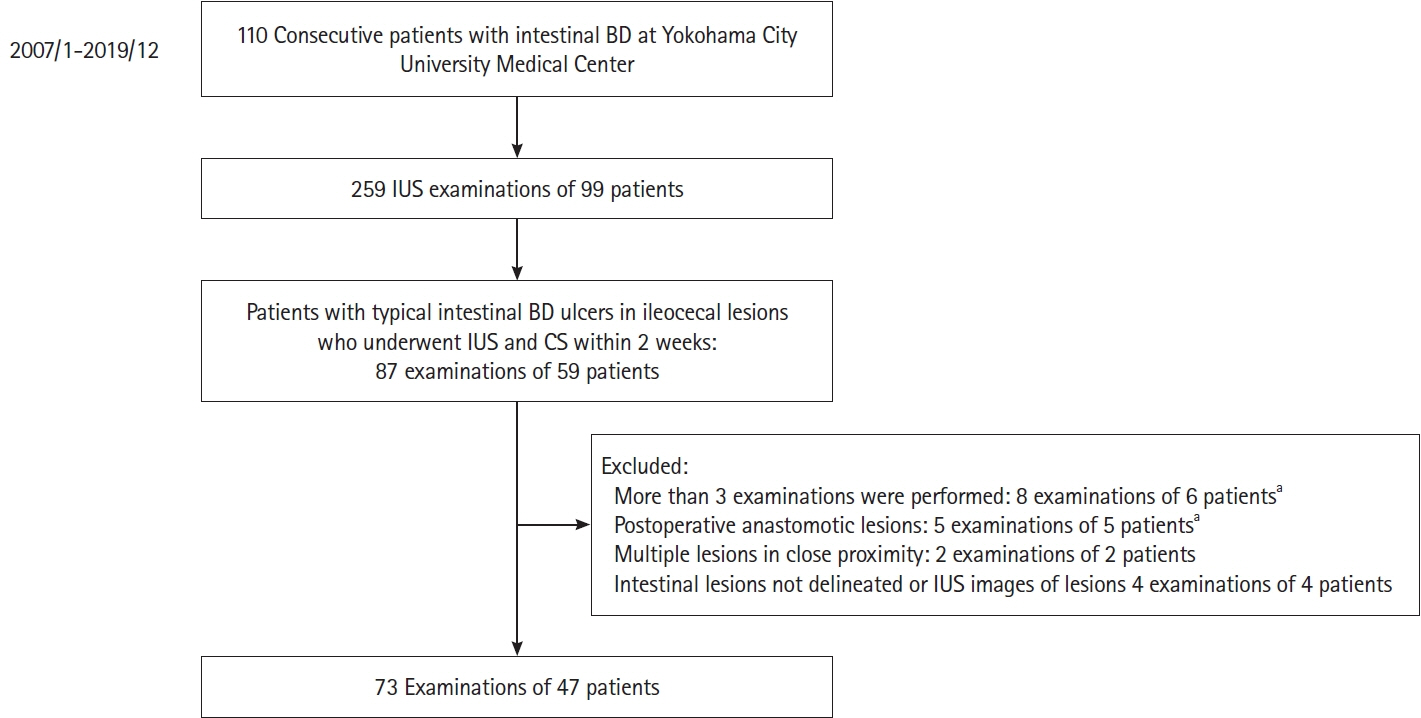
We retrospectively compared intestinal ultrasound findings with 73 corresponding endoscopic images and 6 resected specimens. The intestinal ultrasound findings were assessed for 7 parameters (bowel wall thickness, vascularity [evaluated using the modified Limberg score with color Doppler], bowel wall stratification, white-plaque sign [strong hyperechogenic lines or spots], mesenteric lymphadenopathy, extramural phlegmons, and fistulas), and endoscopic ulcer activity was classified into active, healing, and scar stages. Histopathological findings were evaluated by consensus among experienced pathologists.
Results
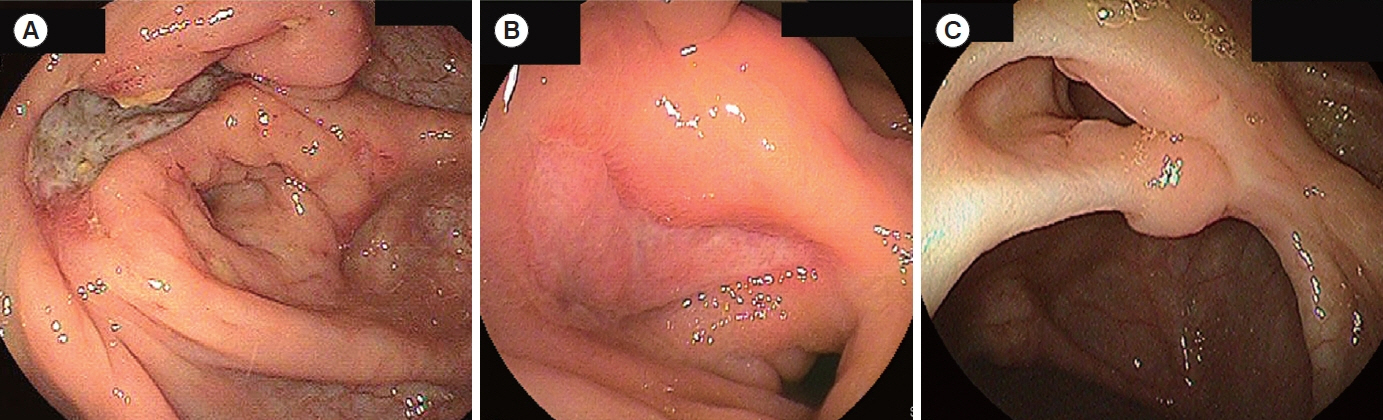
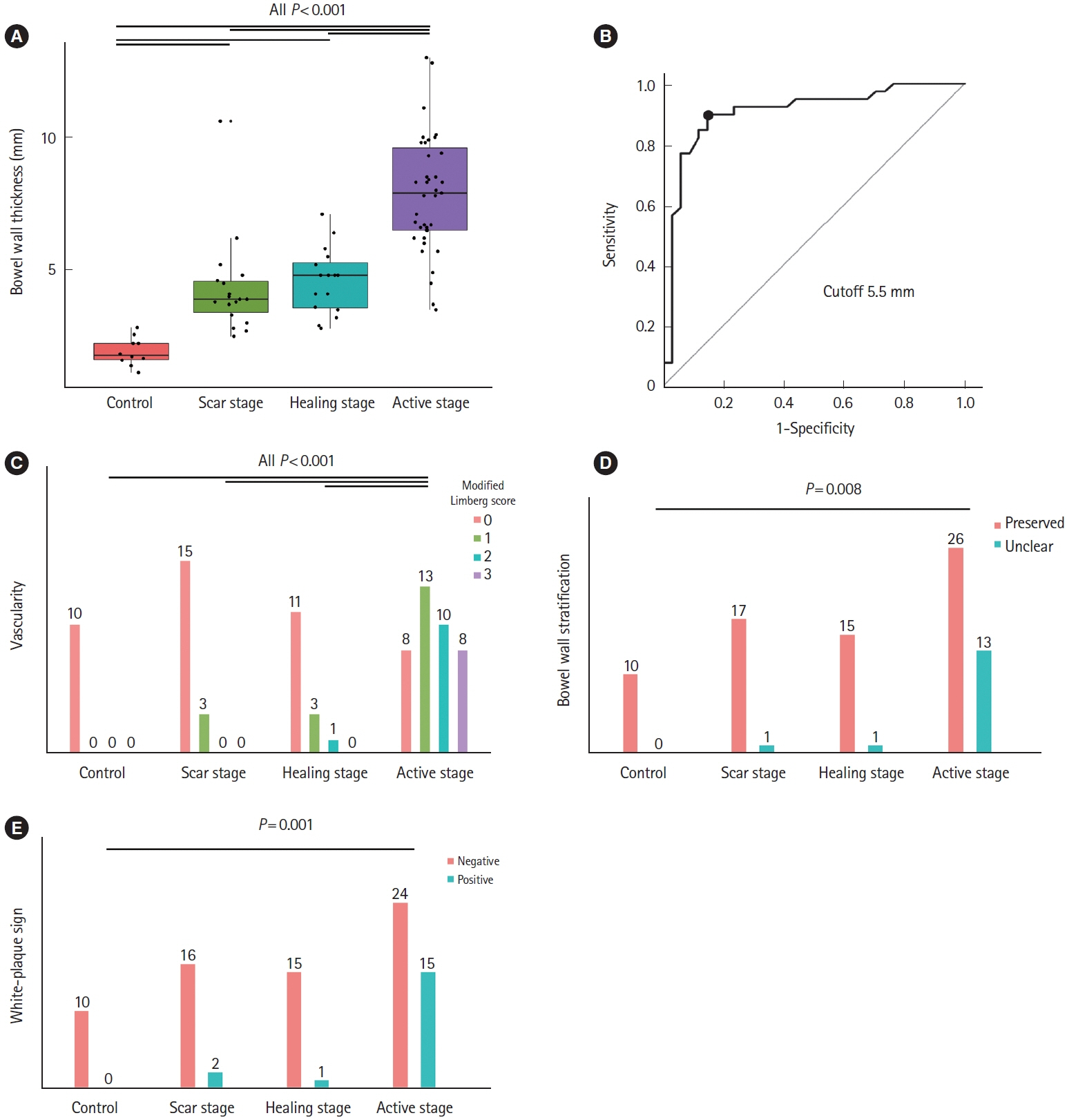
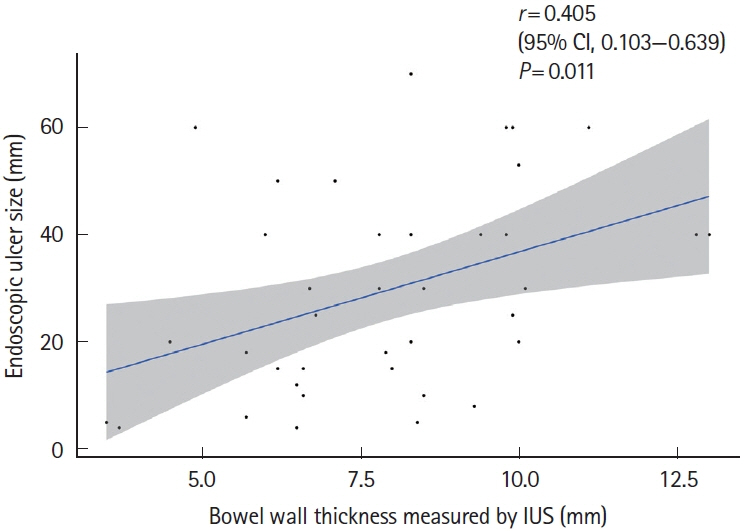
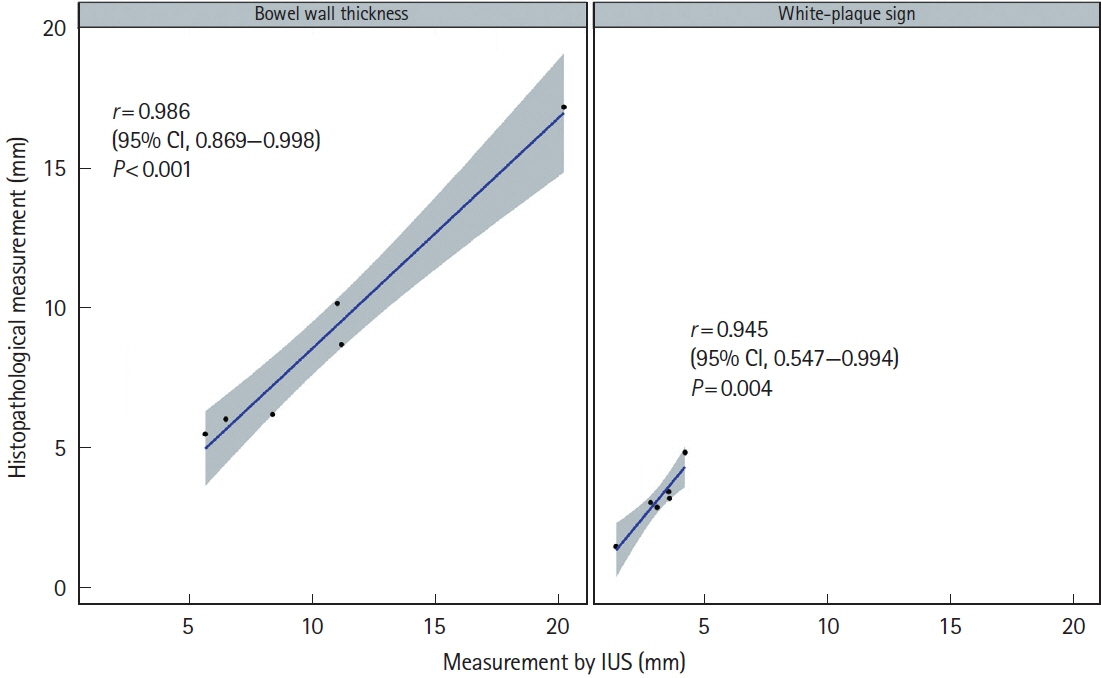
Bowel wall thickness (P< 0.001), vascularity (P< 0.001), loss of bowel wall stratification (P= 0.015), and white-plague sign (P= 0.013) were significantly exacerbated in the endoscopic active ulcer stage. Receiver operating characteristic curve analysis revealed that a bowel wall thickness of > 5.5 mm (sensitivity 89.7%, specificity 85.3%) was potentially useful for detecting active lesions. When compared with histopathological findings, an increase in bowel wall thickness reflected the ulcer marginal ridge, and the white-plaque sign reflected the ulcer bottom.
Conclusions
Intestinal ultrasound is useful for monitoring intestinal ulcer activity in intestinal Behçet disease.
Keyword
Figure
Reference
-
1. Sakane T, Takeno M, Suzuki N, Inaba G. Behçet’s disease. N Engl J Med. 1999; 341:1284–1291.
Article2. James DG. Behcet’s syndrome. N Engl J Med. 1979; 301:431–432.3. He K, Yan X, Wu D. Intestinal Behcet’s disease: a review of the immune mechanism and present and potential biological agents. Int J Mol Sci. 2023; 24:8176.
Article4. Skef W, Hamilton MJ, Arayssi T. Gastrointestinal Behçet’s disease: a review. World J Gastroenterol. 2015; 21:3801–3812.
Article5. Hatemi I, Esatoglu SN, Hatemi G, Erzin Y, Yazici H, Celik AF. Characteristics, treatment, and long-term outcome of gastrointestinal involvement in Behcet’s syndrome: a strobe-compliant observational study from a dedicated multidisciplinary center. Medicine (Baltimore). 2016; 95:e3348.6. Nguyen A, Upadhyay S, Javaid MA, et al. Behcet’s disease: an in-depth review about pathogenesis, gastrointestinal manifestations, and management. Inflamm Intest Dis. 2021; 6:175–185.
Article7. Watanabe K, Tanida S, Inoue N, et al. Evidence-based diagnosis and clinical practice guidelines for intestinal Behçet’s disease 2020 edited by Intractable Diseases, the Health and Labour Sciences Research Grants. J Gastroenterol. 2020; 55:679–700.
Article8. Cheon JH, Kim WH. An update on the diagnosis, treatment, and prognosis of intestinal Behçet’s disease. Curr Opin Rheumatol. 2015; 27:24–31.
Article9. Hatemi G, Christensen R, Bang D, et al. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis. 2018; 77:808–818.
Article10. He K, Wu D. The treatment principles and targets for intestinal Behcet’s disease. Therap Adv Gastroenterol. 2023; 16:17562848231167283.
Article11. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019; 13:144–164.
Article12. Kucharzik T, Wittig BM, Helwig U, et al. Use of intestinal ultrasound to monitor Crohn’s disease activity. Clin Gastroenterol Hepatol. 2017; 15:535–542.
Article13. Kucharzik T, Tielbeek J, Carter D, et al. ECCO-ESGAR topical review on optimizing reporting for cross-sectional imaging in inflammatory bowel disease. J Crohns Colitis. 2022; 16:523–543.
Article14. Rimola J, Torres J, Kumar S, Taylor SA, Kucharzik T. Recent advances in clinical practice: advances in cross-sectional imaging in inflammatory bowel disease. Gut. 2022; 71:2587–2597.
Article15. Ilvemark JF, Hansen T, Goodsall TM, et al. Defining transabdominal intestinal ultrasound treatment response and remission in inflammatory bowel disease: systematic review and expert consensus statement. J Crohns Colitis. 2022; 16:554–580.
Article16. Peker E, Erden A, Erden İ, Düzgün N. Intestinal Behçet disease: evaluation with MR enterography: a case-control study. AJR Am J Roentgenol. 2018; 211:767–775.
Article17. You JK, Kim MJ, Park S, Chung JJ, Kim WH. Intestinal Behçet’s disease: breath-hold MR imaging. Abdom Imaging. 2001; 26:309–314.
Article18. Yang H, Zhang H, Liu W, et al. Computed tomography enterography increases the ability of endoscopy to differentiate Crohn’s disease from intestinal Behçet’s disease. Front Med (Lausanne). 2022; 9:900458.
Article19. Park MJ, Lim JS. Computed tomography enterography for evaluation of inflammatory bowel disease. Clin Endosc. 2013; 46:327–366.
Article20. Ma L, Wang M, Li W, et al. Pilot case-control study to explore the value of intestinal ultrasound in the differentiation of two common diseases involving the ileocecal region: intestinal Behçet’s disease and Crohn’s disease. Quant Imaging Med Surg. 2021; 11:3200–3208.21. Cheon JH, Kim ES, Shin SJ, et al. Development and validation of novel diagnostic criteria for intestinal Behçet’s disease in Korean patients with ileocolonic ulcers. Am J Gastroenterol. 2009; 104:2492–2499.
Article22. Maconi G, Nylund K, Ripolles T, et al. EFSUMB recommendations and clinical guidelines for intestinal ultrasound (GIUS) in inflammatory bowel diseases. Ultraschall Med. 2018; 39:304–317.
Article23. Kaneko E, Hoshihara Y, Sakaki N, et al. Peptic ulcer recurrence during maintenance therapy with H2-receptor antagonist following first-line therapy with proton pump inhibitor. J Gastroenterol. 2000; 35:824–831.
Article24. Tanida S, Inoue N, Kobayashi K, et al. Adalimumab for the treatment of Japanese patients with intestinal Behçet’s disease. Clin Gastroenterol Hepatol. 2015; 13:940–948.
Article25. Limberg B. Diagnosis of chronic inflammatory bowel disease by ultrasonography. Z Gastroenterol. 1999; 37:495–508.26. Sasaki T, Kunisaki R, Kinoshita H, et al. Doppler ultrasound findings correlate with tissue vascularity and inflammation in surgical pathology specimens from patients with small intestinal Crohn’s disease. BMC Res Notes. 2014; 7:363.
Article27. Taylor SA, Avni F, Cronin CG, et al. The first joint ESGAR/ ESPR consensus statement on the technical performance of crosssectional small bowel and colonic imaging. Eur Radiol. 2017; 27:2570–2582.
Article28. Ripollés T, Martínez-Pérez MJ, Paredes JM, Vizuete J, GarcíaMartínez E, Jiménez-Restrepo DH. Contrast-enhanced ultrasound in the differentiation between phlegmon and abscess in Crohn’s disease and other abdominal conditions. Eur J Radiol. 2013; 82:e525–e531.
Article29. Kunihiro K, Hata J, Haruma K, Manabe N, Tanaka S, Chayama K. Sonographic detection of longitudinal ulcers in Crohn disease. Scand J Gastroenterol. 2004; 39:322–326.
Article30. Wang F, Numata K, Yonezawa H, et al. Consistency of trans-abdominal and water-immersion ultrasound images of diseased intestinal segments in Crohn’s disease. Diagnostics (Basel). 2020; 10:267.
Article31. De Voogd F, Wilkens R, Gecse K, et al. A reliability study: strong inter-observer agreement of an expert panel for intestinal ultrasound in ulcerative colitis. J Crohns Colitis. 2021; 15:1284–1290.
Article32. Sagami S, Kobayashi T, Miyatani Y, et al. Accuracy of ultrasound for evaluation of colorectal segments in patients with inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021; 19:908–921.
Article33. Lee CR, Kim WH, Cho YS, et al. Colonoscopic findings in intestinal Behçet’s disease. Inflamm Bowel Dis. 2001; 7:243–249.
Article34. Bhatnagar G, Quinn L, Higginson A, et al. Observer agreement for small bowel ultrasound in Crohn’s disease: results from the METRIC trial. Abdom Radiol (NY). 2020; 45:3036–3045.
Article35. Kinoshita K, Katsurada T, Nishida M, et al. Usefulness of transabdominal ultrasonography for assessing ulcerative colitis: a prospective, multicenter study. J Gastroenterol. 2019; 54:521–529.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intestinal Behcet's disease in a child: a case report
- Advances in Management of Intestinal Behçet’s Disease: A Perspective From Gastroenterologists
- Optimal diagnosis and disease activity monitoring of intestinal Behçet's disease
- Diagnosis of Intestinal Behcet's Disease
- Update on the Treatment of Intestinal Behcet's Disease