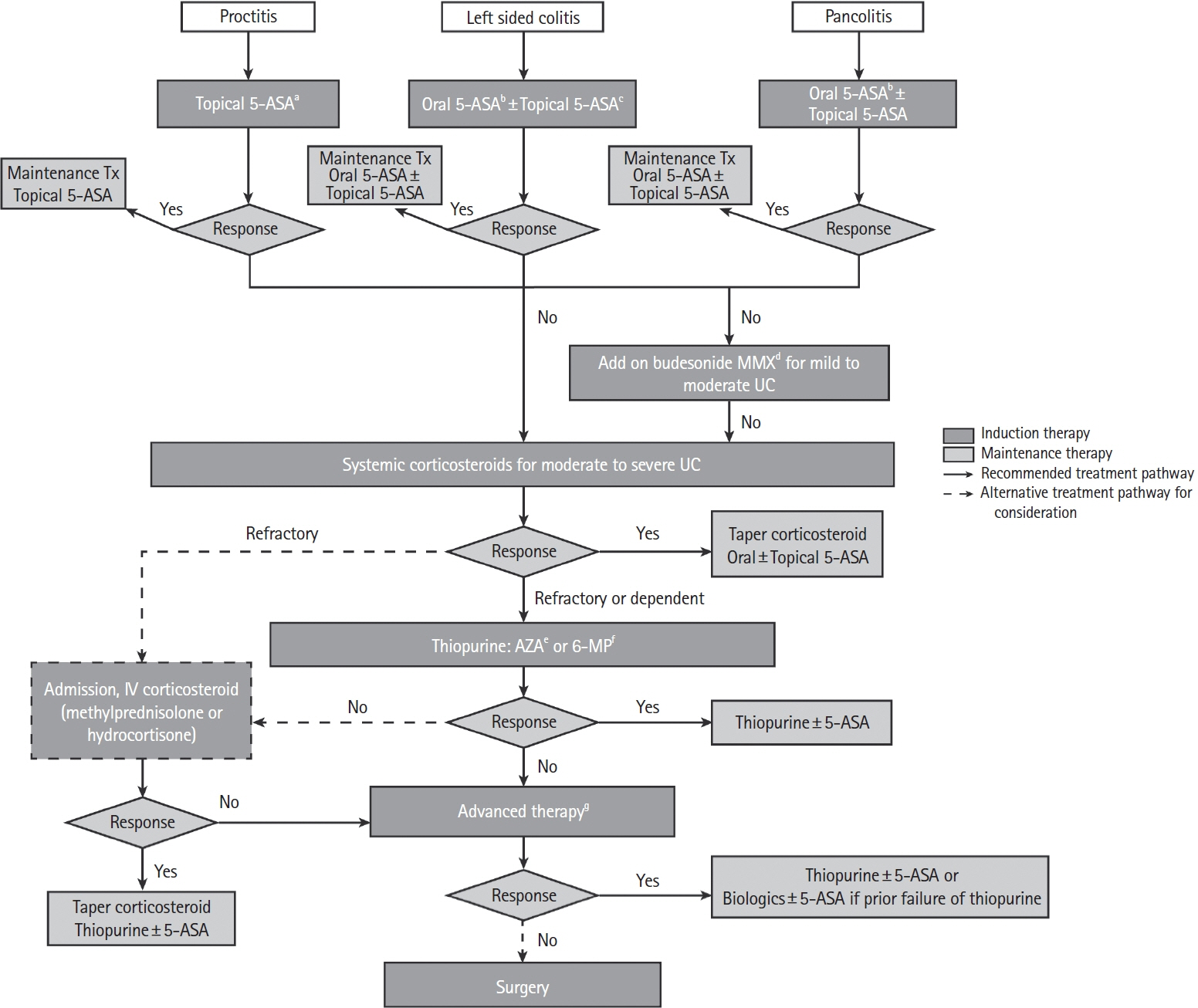
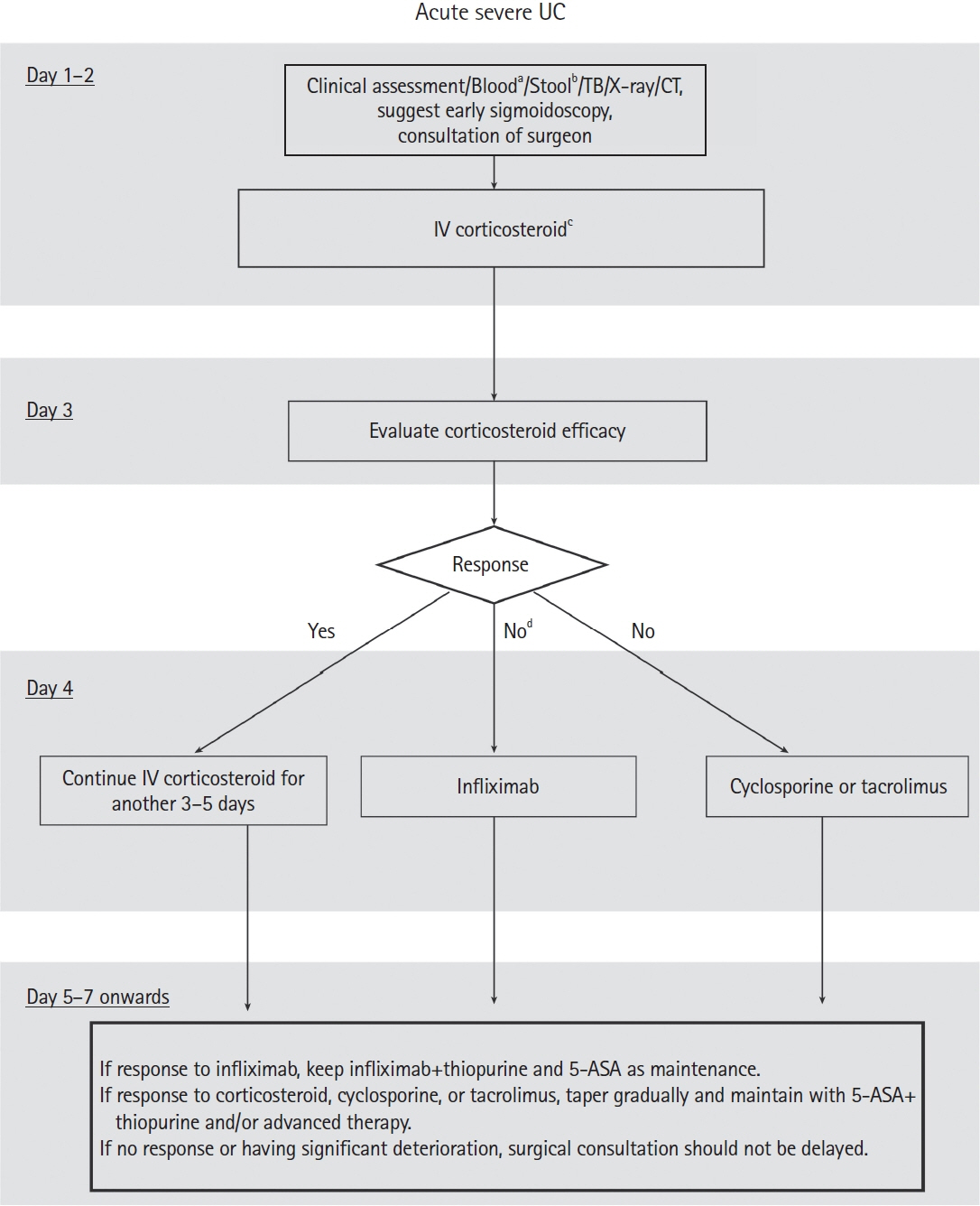
Intest Res.
2024 Jul;22(3):213-249. 10.5217/ir.2023.00050.
Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease updated in 2023
- Affiliations
-
- 1Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan
- 22 Division of Gastroenterology, Changhua Christian Hospital, Changhua, Taiwan
- 3Department of Pediatrics, National Taiwan University Hospital, Taipei, Taiwan
- 4Division of Gastroenterology, Department of Internal Medicine, MacKay Memorial Hospital, Taipei, Taiwan
- 5MacKay Junior College of Medicine, Nursing and Management, Taipei, Taiwan
- 6MacKay Medical College, Taipei, Taiwan
- 7Department of Pathology, Taipei City Hospital, Renai-Branch, Taipei, Taiwan
- 8Division of Gastroenterology and Hepatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
- 9Division of Colon and Rectal Surgery, Department of Surgery, Chiayi and Wangiao Branch, Taichung Veterans General Hospital, Taichung, Taiwan
- 10Center for Digestive Medicine, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan
- 11School of Chinese Medicine, China Medical University, Taichung, Taiwan
- 12Division of General Surgery, Department of Surgery, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan
- 13Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 14Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 15Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung, Taiwan
- 16Division of Colon and Rectal Surgery, Department of Surgery, MacKay Memorial Hospital, MacKay Medical College, Taipei, Taiwan
- 17Division of Gastroenterology and Hepatology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 18Department of Gastroenterology and Hepatology, Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan
- 19Chang Gung Microbiota Therapy Center, Linkou Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan
- 20Inflammatory Bowel Disease Center, Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan
- 21Division of Gastroenterology and Hepatology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan
- 22Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 23School of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 24Division of Colon and Rectal Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
- 25Department of Surgery, Faculty of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 26Department of Pediatrics, National Taiwan University College of Medicine and Children’s Hospital, Taipei, Taiwan
- 27Department of Oncology, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
- 28Department of Medical Imaging, National Taiwan University Hospital, Taipei, Taiwan
- 29Department of Forensic Medicine and Pathology, National Taiwan University Hospital, Taipei, Taiwan
- 30Department of Pathology, Good Liver Clinic, Taipei, Taiwan
- 31Division of Gastroenterology and Hepatology, Department of Internal Medicine, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
- 32School of Medicine, College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 33Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 34Department of Medical Research, National Taiwan University Hospital, Hsin-Chu Branch, Hsin-Chu, Taiwan
- 35Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Gangshan Hospital, Kaohsiung, Taiwan
- 36Department of Medicine, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 37Regenerative Medicine and Cell Therapy Research Center, Kaohsiung Medical University, Kaohsiung, Taiwan
- KMID: 2558187
- DOI: http://doi.org/10.5217/ir.2023.00050
Abstract
- Ulcerative colitis (UC) is a chronic inflammation of the gastrointestinal tract and is characterized by alternating periods of inflammation and remission. Although UC incidence is lower in Taiwan than in Western countries, its impact remains considerable, demanding updated guidelines for addressing local healthcare challenges and patient needs. The revised guidelines employ international standards and recent research, emphasizing practical implementation within the Taiwanese healthcare system. Since the inception of the guidelines in 2017, the Taiwan Society of Inflammatory Bowel Disease has acknowledged the need for ongoing revisions to incorporate emerging therapeutic options and evolving disease management practices. This updated guideline aims to align UC management with local contexts, ensuring comprehensive and context-specific recommendations, thereby raising the standard of care for UC patients in Taiwan. By adapting and optimizing international protocols for local relevance, these efforts seek to enhance health outcomes for patients with UC.
Keyword
Figure
Reference
-
1. Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc. 2014; 89:1553–1563.2. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012; 142:46–54.3. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011; 140:1785–1794.
Article4. Wei SC, Lin MH, Tung CC, et al. A nationwide populationbased study of the inflammatory bowel diseases between 1998 and 2008 in Taiwan. BMC Gastroenterol. 2013; 13:166.
Article5. Chuang CH, Lin SH, Chen CY, Sheu BS, Kao AW, Wang JD. Increasing incidence and lifetime risk of inflammatory bowel disease in Taiwan: a nationwide study in a low-endemic area 1998-2010. Inflamm Bowel Dis. 2013; 19:2815–2819.
Article6. Kuo CJ, Yu KH, See LC, et al. The trend of inflammatory bowel diseases in taiwan: a population-based study. Dig Dis Sci. 2015; 60:2454–2462.
Article7. Wang LH, Yang YJ, Cheng WC, Wang WM, Lin SH, Shieh CC. Higher risk for hematological malignancies in inflammatory bowel disease: a nationwide population-based study in Taiwan. Am J Gastroenterol. 2016; 111:1313–1319.
Article8. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011; 365:1713–1725.
Article9. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012; 6:965–990.
Article10. Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012; 6:991–1030.
Article11. Talley NJ, Abreu MT, Achkar JP, et al. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol. 2011; 106 Suppl 1:S2–S25.
Article12. Ooi CJ, Fock KM, Makharia GK, et al. The Asia-Pacific consensus on ulcerative colitis. J Gastroenterol Hepatol. 2010; 25:453–468.
Article13. Lan JY. Achieving and sustaining universal health coverage: fiscal reform of the National Health Insurance in Taiwan. Appl Health Econ Health Policy. 2017; 15:717–731.
Article14. Chen NY, Chuang CH, Chang YC, Kao Yang YH, Chen PH, Cheng CL. Suboptimal outcomes and retreatment rate of patients with Crohn’s disease after forced discontinuation of biologics: a nationwide population-based study. Clin Pharmacol Ther. 2023; 114:914–921.15. Yen HH, Hsu YC, Kuo CH, Hsu TC, Chen YY. Real‐world experience of adalimumab therapy for patients with ulcerative colitis: a single tertiary medical center experience in Central Taiwan. Adv Dig Med. 2023; 10:28–33.
Article16. Wei SC, Chang TA, Chao TH, et al. Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease. Intest Res. 2017; 15:266–284.
Article17. Yen HH, Weng MT, Tung CC, et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide populationbased study. Intest Res. 2019; 17:54–62.
Article18. Leddin D, Tamim H, Levy AR. Decreasing incidence of inflammatory bowel disease in eastern Canada: a population database study. BMC Gastroenterol. 2014; 14:140.
Article19. Lophaven SN, Lynge E, Burisch J. The incidence of inflammatory bowel disease in Denmark 1980-2013: a nationwide cohort study. Aliment Pharmacol Ther. 2017; 45:961–972.
Article20. van den Heuvel TR, Jeuring SF, Zeegers MP, et al. A 20-year temporal change analysis in incidence, presenting phenotype and mortality, in the Dutch IBDSL cohort: can diagnostic factors explain the increase in IBD incidence? J Crohns Colitis. 2017; 11:1169–1179.
Article21. Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: east meets west. J Gastroenterol Hepatol. 2020; 35:380–389.
Article22. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017; 152:313–321.
Article23. Shah SC, Khalili H, Gower-Rousseau C, et al. Sex-based differences in incidence of inflammatory bowel diseases: pooled analysis of population-based studies from western countries. Gastroenterology. 2018; 155:1079–1089.
Article24. Zeng Z, Zhu Z, Yang Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. 2013; 28:1148–1153.
Article25. Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015; 21:623–630.
Article26. Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009; 44:659–665.
Article27. Ng SC, Tsoi KK, Kamm MA, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis. 2012; 18:1164–1176.28. Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008; 57:1185–1191.
Article29. Feuerstein JD, Moss AC, Farraye FA. Ulcerative colitis. Mayo Clin Proc. 2019; 94:1357–1373.
Article30. Karreman MC, Luime JJ, Hazes JM, Weel AE. The prevalence and incidence of axial and peripheral spondyloarthritis in inflammatory bowel disease: a systematic review and metaanalysis. J Crohns Colitis. 2017; 11:631–642.
Article31. Chou JW, Lai HC, Chang CH, Cheng KS, Feng CL, Chen TW. Epidemiology and clinical outcomes of inflammatory bowel disease: a hospital-based study in central Taiwan. Gastroenterol Res Pract. 2019; 2019:4175923.
Article32. Hsu YC, Wu TC, Lo YC, Wang LS. Gastrointestinal complications and extraintestinal manifestations of inflammatory bowel disease in Taiwan: a population-based study. J Chin Med Assoc. 2017; 80:56–62.
Article33. Burisch J, Katsanos KH, Christodoulou DK, et al. Natural disease course of ulcerative colitis during the first five years of follow-up in a European population-based inception cohort: an epi-IBD study. J Crohns Colitis. 2019; 13:198–208.
Article34. Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2019; 68:423–433.35. Ibrahim TM, Iheonunekwu N, Gill V, Vantapool H. Differentiating amoebic ulcero-haemorrhagic recto-colitis from idiopathic inflammatory bowel disease: still a diagnostic dilemma. West Indian Med J. 2005; 54:210–212.
Article36. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019; 68(Suppl 3):s1–s106.
Article37. Truelove SC, Witts LJ. Cortisone in ulcerative colitis: final report on a therapeutic trial. Br Med J. 1955; 2:1041–1048.38. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987; 317:1625–1629.
Article39. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017; 11:3–25.
Article40. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017; 11:649–670.
Article41. Hsiao PY, Weng MT, Chang CH, et al. Anemia in inflammatory bowel disease course is associated with patients’ worse outcome. J Formos Med Assoc. 2023; 122:549–556.
Article42. Jukic A, Bakiri L, Wagner EF, Tilg H, Adolph TE. Calprotectin: from biomarker to biological function. Gut. 2021; 70:1978–1988.
Article43. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019; 13:144–164.
Article44. Paine ER. Colonoscopic evaluation in ulcerative colitis. Gastroenterol Rep (Oxf). 2014; 2:161–168.
Article45. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389:1756–1770.
Article46. Navaneethan U, Kochhar G, Phull H, et al. Severe disease on endoscopy and steroid use increase the risk for bowel perforation during colonoscopy in inflammatory bowel disease patients. J Crohns Colitis. 2012; 6:470–475.
Article47. Choi YS, Kim JK, Kim WJ. Clinical characteristics and prognosis of patients with ulcerative colitis that shows rectal sparing at initial diagnosis. World J Gastrointest Endosc. 2021; 13:407–415.48. Kleer CG, Appelman HD. Ulcerative colitis: patterns of involvement in colorectal biopsies and changes with time. Am J Surg Pathol. 1998; 22:983–989.49. Bernstein CN, Shanahan F, Anton PA, Weinstein WM. Patchiness of mucosal inflammation in treated ulcerative colitis: a prospective study. Gastrointest Endosc. 1995; 42:232–237.
Article50. Park SH, Yang SK, Park SK, et al. Atypical distribution of inflammation in newly diagnosed ulcerative colitis is not rare. Can J Gastroenterol Hepatol. 2014; 28:125–130.
Article51. Joo M, Odze RD. Rectal sparing and skip lesions in ulcerative colitis: a comparative study of endoscopic and histologic findings in patients who underwent proctocolectomy. Am J Surg Pathol. 2010; 34:689–696.
Article52. Deepak P, Bruining DH. Radiographical evaluation of ulcerative colitis. Gastroenterol Rep (Oxf). 2014; 2:169–177.
Article53. Pola S, Patel D, Ramamoorthy S, et al. Strategies for the care of adults hospitalized for active ulcerative colitis. Clin Gastroenterol Hepatol. 2012; 10:1315–1325.
Article54. Buckell NA, Williams GT, Bartram CI, Lennard-Jones JE. Depth of ulceration in acute colitis: correlation with outcome and clinical and radiologic features. Gastroenterology. 1980; 79:19–25.55. Imbriaco M, Balthazar EJ. Toxic megacolon: role of CT in evaluation and detection of complications. Clin Imaging. 2001; 25:349–354.
Article56. Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013; 7:556–585.
Article57. Nardone OM, Calabrese G, Testa A, et al. The impact of intestinal ultrasound on the management of inflammatory bowel disease: from established facts toward new horizons. Front Med (Lausanne). 2022; 9:898092.
Article58. Lin WC, Chang CW, Chen MJ, Wang HY. Intestinal ultrasound in inflammatory bowel disease: a novel and increasingly important tool. J Med Ultrasound. 2023; 31:86–91.
Article59. Sagami S, Kobayashi T, Miyatani Y, et al. Accuracy of ultrasound for evaluation of colorectal segments in patients with inflammatory bowel diseases: a systematic review and metaanalysis. Clin Gastroenterol Hepatol. 2021; 19:908–921.
Article60. Allocca M, Fiorino G, Bonovas S, et al. Accuracy of humanitas ultrasound criteria in assessing disease activity and severity in ulcerative colitis: a prospective study. J Crohns Colitis. 2018; 12:1385–1391.
Article61. Allocca M, Filippi E, Costantino A, et al. Milan ultrasound criteria are accurate in assessing disease activity in ulcerative colitis: external validation. United European Gastroenterol J. 2021; 9:438–442.
Article62. Bots S, Nylund K, Löwenberg M, Gecse K, D’Haens G. Intestinal ultrasound to assess disease activity in ulcerative colitis: development of a novel UC-Ultrasound Index. J Crohns Colitis. 2021; 15:1264–1271.63. Washington K, Greenson JK, Montgomery E, et al. Histopathology of ulcerative colitis in initial rectal biopsy in children. Am J Surg Pathol. 2002; 26:1441–1449.
Article64. Schmitz-Moormann P, Himmelmann GW. Does quantitative histology of rectal biopsy improve the differential diagnosis of Crohn’s disease and ulcerative colitis in adults? Pathol Res Pract. 1988; 183:481–488.
Article65. DeRoche TC, Xiao SY, Liu X. Histological evaluation in ulcerative colitis. Gastroenterol Rep (Oxf). 2014; 2:178–192.
Article66. Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, et al. Development and validation of the Nancy histological index for UC. Gut. 2017; 66:43–49.
Article67. Magro F, Doherty G, Peyrin-Biroulet L, et al. ECCO position paper: harmonization of the approach to ulcerative colitis histopathology. J Crohns Colitis. 2020; 14:1503–1511.
Article68. Bharadwaj S, Narula N, Tandon P, Yaghoobi M. Role of endoscopy in inflammatory bowel disease. Gastroenterol Rep (Oxf). 2018; 6:75–82.
Article69. Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016; 388:1081–1088.
Article70. World Health Organization. Global hepatitis report, 2017 [Internet]. c2024. [cited 2024 Apr 1]. https://www.who.int/publications/i/item/9789241565455.71. Lin WC, Tai WC, Chang CH, et al. Real-world evidence of effectiveness and safety of vedolizumab for inflammatory bowel disease in Taiwan: a prospective nationwide registry (VIOLET) study. Inflamm Bowel Dis. 2023; 29:1730–1740.
Article72. Giri S, Agrawal D, Afzalpurkar S, et al. Prevalence of hepatitis B virus and hepatitis C virus infection in patients with inflammatory bowel disease: a systematic review and meta-analysis. Intest Res. 2023; 21:392–405.
Article73. Ran Z, Wu K, Matsuoka K, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology practice recommendations for medical management and monitoring of inflammatory bowel disease in Asia. J Gastroenterol Hepatol. 2021; 36:637–645.
Article74. Rahier JF, Magro F, Abreu C, et al. Second European evidencebased consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014; 8:443–468.
Article75. Beaugerie L, Rahier JF, Kirchgesner J. Predicting, preventing, and managing treatment-related complications in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020; 18:1324–1335.
Article76. Loras C, Gisbert JP, Mínguez M, et al. Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut. 2010; 59:1340–1346.
Article77. Park SH, Yang SK, Lim YS, et al. Clinical courses of chronic hepatitis B virus infection and inflammatory bowel disease in patients with both diseases. Inflamm Bowel Dis. 2012; 18:2004–2010.
Article78. Axiaris G, Zampeli E, Michopoulos S, Bamias G. Management of hepatitis B virus infection in patients with inflammatory bowel disease under immunosuppressive treatment. World J Gastroenterol. 2021; 27:3762–3779.
Article79. National Health Insurance Administration. Drug Reimbursement Regulations: Section 10 Antimicrobial Agents. Taipei: National Health Insurance Administration;2023.80. Olivera PA, Lasa JS, Zubiaurre I, et al. Opportunistic infections in patients with inflammatory bowel disease treated with advanced therapies: a systematic review and meta-analysis of randomized controlled trials. J Crohns Colitis. 2023; 17:199–210.
Article81. Weng MT, Wei SC, Lin CC, et al. Seminar report from the 2014 Taiwan Society of Inflammatory Bowel Disease (TSIBD) spring forum (May 24th, 2014): Crohn’s disease versus intestinal tuberculosis infection. Intest Res. 2015; 13:6–10.
Article82. Lai HC, Chang CH, Cheng KS, Chen TW, Tsai YY, Chou JW. QuantiFERON-TB gold test conversion is associated with active tuberculosis development in inflammatory bowel disease patients treated with biological agents: an experience of a medical center in Taiwan. Gastroenterol Res Pract. 2019; 2019:7132875.
Article83. Horsburgh CR Jr, Rubin EJ. Clinical practice: latent tuberculosis infection in the United States. N Engl J Med. 2011; 364:1441–1448.84. Taiwan Centers for Disease Control. Taiwan guidelines for TB diagnosis & treatment. Taipei: Taiwan Centers for Disease Control, Ministry of Health and Welfare;2017.85. Fehily SR, Al-Ani AH, Abdelmalak J, et al. Review article: latent tuberculosis in patients with inflammatory bowel diseases receiving immunosuppression-risks, screening, diagnosis and management. Aliment Pharmacol Ther. 2022; 56:6–27.86. Jiang HY, Wang SY, Deng M, et al. Immune response to hepatitis B vaccination among people with inflammatory bowel diseases: a systematic review and meta-analysis. Vaccine. 2017; 35:2633–2641.
Article87. Lai SW, Liao KF, Lin CL, Kuo YH, Liu CS, Hwang BF. The incidence rate of herpes zoster in inflammatory bowel disease: a meta-analysis of cohort studies. Medicine (Baltimore). 2021; 100:e26863.88. Din S, Selinger CP, Black CJ, Ford AC. Systematic review with network meta-analysis: risk of herpes zoster with biological therapies and small molecules in inflammatory bowel disease. Aliment Pharmacol Ther. 2023; 57:666–675.
Article89. Kucharzik T, Ellul P, Greuter T, et al. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021; 15:879–913.
Article90. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017; 112:241–258.
Article91. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018; 67:103–108.
Article92. Lee YJ, Kim ES. Vaccination strategies for Korean patients with inflammatory bowel disease. Korean J Intern Med. 2022; 37:920–930.
Article93. Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68:698–702.
Article94. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–753.
Article95. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005; 19 Suppl A:5A–36A.
Article96. Dotson JL, Crandall WV, Zhang P, et al. Feasibility and validity of the pediatric ulcerative colitis activity index in routine clinical practice. J Pediatr Gastroenterol Nutr. 2015; 60:200–204.
Article97. Balestrieri P, Ribolsi M, Guarino MP, Emerenziani S, Altomare A, Cicala M. Nutritional aspects in inflammatory bowel diseases. Nutrients. 2020; 12:372.
Article98. Forbes A, Escher J, Hébuterne X, et al. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017; 36:321–347.99. Bischoff SC, Escher J, Hébuterne X, et al. ESPEN practical guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2020; 39:632–653.
Article100. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019; 114:384–413.
Article101. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017; 11:769–784.
Article102. Bressler B, Marshall JK, Bernstein CN, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015; 148:1035–1058.
Article103. Burri E, Maillard MH, Schoepfer AM, et al. Treatment algorithm for mild and moderate-to-severe ulcerative colitis: an update. Digestion. 2020; 101 Suppl 1:2–15.
Article104. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.
Article105. Stoner PL, Kamel A, Ayoub F, et al. Perioperative care of patients with inflammatory bowel disease: focus on nutritional support. Gastroenterol Res Pract. 2018; 2018:7890161.106. Appropriate use of gastrointestinal endoscopy. Gastrointest Endosc. 2000; 52(6 Pt 1):831–837.107. Salinas H, Dursun A, Konstantinidis I, et al. Does preoperative total parenteral nutrition in patients with ulcerative colitis produce better outcomes? Int J Colorectal Dis. 2012; 27:1479–1483.
Article108. Schwartz E. Perioperative parenteral nutrition in adults with inflammatory bowel disease: a review of the literature. Nutr Clin Pract. 2016; 31:159–170.
Article109. Hsieh CT, Weng MT, Tung CC, et al. Dietary beliefs and information resources of ulcerative colitis patients in clinical remission: a cross-sectional survey in Taiwan. Clin Nutr ESPEN. 2022; 51:430–436.
Article110. Marshall JK, Thabane M, Steinhart AH, Newman JR, Anand A, Irvine EJ. Rectal 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2010; (1):CD004115.
Article111. Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006; (2):CD000543.
Article112. Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012; 10:CD000543.113. Parisian KR, Lashner BA. Management of steroid-dependent and steroid-refractory ulcerative colitis. In: Lichtenstein GR, ed. Medical therapy of ulcerative colitis. New York: Springer, 2014:313-320.114. Wang Y, Parker CE, Feagan BG, MacDonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016; 2016:CD000544.
Article115. Taylor K, Gibson PR. Conventional therapy of ulcerative colitis: corticosteroids. In: Baumgart DC, ed. Crohn’s disease and ulcerative colitis. New York: Springer, 2017:399-412.116. Barrett K, Saxena S, Pollok R. Using corticosteroids appropriately in inflammatory bowel disease: a guide for primary care. Br J Gen Pract. 2018; 68:497–498.
Article117. Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011; 60:571–607.
Article118. Timmer A, McDonald JW, Tsoulis DJ, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012; (9):CD000478.
Article119. Rogler G, König A, Vavricka SR. Special issue of digestion: inflammatory bowel disease. Digestion. 2020; 101 Suppl 1:1.
Article120. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476.
Article121. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012; 142:257–265.
Article122. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146:96–109.
Article123. Hibi T, Imai Y, Senoo A, Ohta K, Ukyo Y. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: a phase 3, double-blind, randomized, placebo-controlled study: (PURSUIT-J study). J Gastroenterol. 2017; 52:1101–1111.
Article124. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; 369:699–710.
Article125. Sandborn WJ, Baert F, Danese S, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020; 158:562–572.
Article126. Sands BE, Peyrin-Biroulet L, Loftus EV Jr, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019; 381:1215–1226.
Article127. Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020; 158:1450–1461.
Article128. Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. Firstand second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020; 18:2179–2191.129. Benson JM, Peritt D, Scallon BJ, et al. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011; 3:535–545.130. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019; 381:1201–1214.
Article131. Efficacy and safety of mirikizumab as maintenance therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-2 Study. Gastroenterol Hepatol (N Y). 2022; 18(7 Suppl 2):3–4.132. D’Amico F, Parigi TL, Fiorino G, Peyrin-Biroulet L, Danese S. Tofacitinib in the treatment of ulcerative colitis: efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol. 2019; 12:1756284819848631.
Article133. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; 376:1723–1736.
Article134. Paschos P, Katsoula A, Giouleme O, et al. Tofacitinib for induction of remission in ulcerative colitis: systematic review and meta-analysis. Ann Gastroenterol. 2018; 31:572–582.135. Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022; 399:2113–2128.
Article136. Napolitano M, D’Amico F, Ragaini E, Peyrin-Biroulet L, Danese S. Evaluating upadacitinib in the treatment of moderate-tosevere active ulcerative colitis: design, development, and potential position in therapy. Drug Des Devel Ther. 2022; 16:1897–1913.
Article137. Ananthakrishnan AN, Kaplan GG, Bernstein CN, et al. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. Lancet Gastroenterol Hepatol. 2022; 7:666–678.138. Feagan BG, Danese S, Loftus EV Jr, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021; 397:2372–2384.
Article139. Sandborn WJ, Feagan BG, D’Haens G, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021; 385:1280–1291.
Article140. Hindryckx P, Jairath V, D’Haens G. Acute severe ulcerative colitis: from pathophysiology to clinical management. Nat Rev Gastroenterol Hepatol. 2016; 13:654–664.
Article141. Nakase H. Acute severe ulcerative colitis: optimal strategies for drug therapy. Gut Liver. 2023; 17:49–57.
Article142. Holvoet T, Lobaton T, Hindryckx P. Optimal management of Acute Severe Ulcerative Colitis (ASUC): challenges and solutions. Clin Exp Gastroenterol. 2021; 14:71–81.
Article143. Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, part 2: acute severe colitis-an evidence-based consensus guideline from the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018; 67:292–310.144. Whaley KG, Rosen MJ. Contemporary medical management of acute severe ulcerative colitis. Inflamm Bowel Dis. 2019; 25:56–66.
Article145. Singh A, Goyal MK, Midha V, et al. Tofacitinib in Acute Severe Ulcerative Colitis (TACOS): a randomized controlled trial. Am J Gastroenterol. 2024; 119:1365–1372.
Article146. Verdon C, Bessissow T, Lakatos PL. Management of acute severe colitis in the era of biologicals and small molecules. J Clin Med. 2019; 8:2169.
Article147. Hindryckx P, Novak G, Vande Casteele N, et al. Review article: dose optimisation of infliximab for acute severe ulcerative colitis. Aliment Pharmacol Ther. 2017; 45:617–630.
Article148. Syal G, Robbins L, Kashani A, et al. Hypoalbuminemia and bandemia predict failure of infliximab rescue therapy in acute severe ulcerative colitis. Dig Dis Sci. 2021; 66:199–205.
Article149. Chao CY, Al Khoury A, Aruljothy A, et al. High-dose infliximab rescue therapy for hospitalized acute severe ulcerative colitis does not improve colectomy: free survival. Dig Dis Sci. 2019; 64:518–523.150. Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015; 13:330–335.
Article151. Van Assche G, D’Haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003; 125:1025–1031.
Article152. Komaki Y, Komaki F, Ido A, Sakuraba A. Efficacy and safety of tacrolimus therapy for active ulcerative colitis; a systematic review and meta-analysis. J Crohns Colitis. 2016; 10:484–494.
Article153. Williams JG, Alam MF, Alrubaiy L, et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2016; 1:15–24.
Article154. Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012; 380:1909–1915.
Article155. Jia X, Guo R, Hu Z, et al. Efficacy of infliximab, cyclosporine and tacrolimus on ulcerative colitis: a meta-analysis. Medicine (Baltimore). 2020; 99:e22894.156. García MJ, Riestra S, Amiot A, et al. Effectiveness and safety of a third-line rescue treatment for acute severe ulcerative colitis refractory to infliximab or ciclosporin (REASUC study). Aliment Pharmacol Ther. 2024; 59:1248–1259.
Article157. Horrigan JM, Louis E, Spinelli A, et al. The real-world global use of patient-reported outcomes for the care of patients with inflammatory bowel disease. Crohns Colitis 360. 2023; 5:otad006.
Article158. Marcovitch L, Focht G, Carmon N, et al. Development and validation of the TUMMY-UC: a patient-reported outcome for pediatric ulcerative colitis. Gastroenterology. 2023; 164:610–618.
Article159. Yen HH, Chen MW, Chang YY, Huang HY, Hsu TC, Chen YY. Predictive values of stool-based tests for mucosal healing among Taiwanese patients with ulcerative colitis: a retrospective cohort analysis. PeerJ. 2020; 8:e9537.
Article160. Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009; 15:1851–1858.161. Jha AK, Chaudhary M, Dayal VM, et al. Optimal cut-off value of fecal calprotectin for the evaluation of ulcerative colitis: an unsolved issue? JGH Open. 2018; 2:207–213.
Article162. Krzystek-Korpacka M, Kempiński R, Bromke M, Neubauer K. Biochemical biomarkers of mucosal healing for inflammatory bowel disease in adults. Diagnostics (Basel). 2020; 10:367.
Article163. Laserna-Mendieta EJ, Lucendo AJ. Faecal calprotectin in inflammatory bowel diseases: a review focused on meta-analyses and routine usage limitations. Clin Chem Lab Med. 2019; 57:1295–1307.
Article164. Yoon JY, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig Dis Sci. 2014; 59:829–837.165. Murdoch T, O’Donnell S, Silverberg MS, Panaccione R. Biomarkers as potential treatment targets in inflammatory bowel disease: a systematic review. Can J Gastroenterol Hepatol. 2015; 29:203–208.
Article166. Stojkovic Lalosevic M, Sokic Milutinovic A, Zaric VM, et al. Intestinal ultrasonography as a tool for monitoring disease activity in patients with ulcerative colitis. Int J Clin Pract. 2022; 2022:3339866.
Article167. de Voogd F, van Wassenaer EA, Mookhoek A, et al. Intestinal ultrasound is accurate to determine endoscopic response and remission in patients with moderate to severe ulcerative colitis: a longitudinal prospective cohort study. Gastroenterology. 2022; 163:1569–1581.
Article168. Maaser C, Petersen F, Helwig U, et al. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut. 2020; 69:1629–1636.
Article169. Wright R, Truelove SR. Serial rectal biopsy in ulcerative colitis during the course of a controlled therapeutic trial of various diets. Am J Dig Dis. 1966; 11:847–857.
Article170. Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007; 133:412–422.
Article171. Sood A, Mahajan R, Singh A, Midha V, Mehta V. Endoscopy for assessment of mucosal healing in ulcerative colitis: time bound or response guided? Intest Res. 2022; 20:297–302.
Article172. Wang H, Fewings I, Bornman L, Shadbolt B, Fadia M, Subramaniam K. Histologic remission (NANCY Index) is superior to endoscopic mucosal healing in predicting relapse free survival in patients with ulcerative colitis in clinical and endoscopic remission. J Clin Gastroenterol. 2023; 57:494–500.
Article173. Lemmens B, Arijs I, Van Assche G, et al. Correlation between the endoscopic and histologic score in assessing the activity of ulcerative colitis. Inflamm Bowel Dis. 2013; 19:1194–1201.
Article174. Mosli MH, Feagan BG, Zou G, et al. Development and validation of a histological index for UC. Gut. 2017; 66:50–58.
Article175. Gui X, Bazarova A, Del Amor R, et al. PICaSSO Histologic Remission Index (PHRI) in ulcerative colitis: development of a novel simplified histological score for monitoring mucosal healing and predicting clinical outcomes and its applicability in an artificial intelligence system. Gut. 2022; 71:889–898.176. Myrelid P, Øresland T. A reappraisal of the ileo-rectal anastomosis in ulcerative colitis. J Crohns Colitis. 2015; 9:433–438.
Article177. Andersson P, Söderholm JD. Surgery in ulcerative colitis: indication and timing. Dig Dis. 2009; 27:335–340.
Article178. Lin CC, Wei SC, Lin BR, et al. A retrospective analysis of 20- year data of the surgical management of ulcerative colitis patients in Taiwan: a study of Taiwan Society of Inflammatory Bowel Disease. Intest Res. 2016; 14:248–257.
Article179. Olecki EJ, Kronfli AP, Stahl KA, King S, C Razavi N, Koltun WA. Stoma-less IPAA is not associated with increased anastomotic leak rate or long-term pouch failure in patients with ulcerative colitis. Dis Colon Rectum. 2022; 65:1342–1350.
Article180. Windsor A, Michetti P, Bemelman W, Ghosh S. The positioning of colectomy in the treatment of ulcerative colitis in the era of biologic therapy. Inflamm Bowel Dis. 2013; 19:2695–2703.
Article181. Spinelli A, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: surgical treatment. J Crohns Colitis. 2022; 16:179–189.
Article182. Gu J, Stocchi L, Remzi F, Kiran RP. Factors associated with postoperative morbidity, reoperation and readmission rates after laparoscopic total abdominal colectomy for ulcerative colitis. Colorectal Dis. 2013; 15:1123–1129.
Article183. Wolff BG, Fleshman JW, Beck DE, Pemberton JH, Wexner SD. The ASCRS Textbook of Colon and Rectal Surgery. New York: Springer;2007.184. Bartels SA, Gardenbroek TJ, Ubbink DT, Buskens CJ, Tanis PJ, Bemelman WA. Systematic review and meta-analysis of laparoscopic versus open colectomy with end ileostomy for nontoxic colitis. Br J Surg. 2013; 100:726–733.
Article185. Marceau C, Alves A, Ouaissi M, Bouhnik Y, Valleur P, Panis Y. Laparoscopic subtotal colectomy for acute or severe colitis complicating inflammatory bowel disease: a case-matched study in 88 patients. Surgery. 2007; 141:640–644.
Article186. Germain A, de Buck van Overstraeten A, Wolthuis A, et al. Outcome of restorative proctocolectomy with an ileo-anal pouch for ulcerative colitis: effect of changes in clinical practice. Colorectal Dis. 2018; 20:O30–O38.
Article187. Swenson BR, Hollenbeak CS, Poritz LS, Koltun WA. Modified two-stage ileal pouch-anal anastomosis: equivalent outcomes with less resource utilization. Dis Colon Rectum. 2005; 48:256–261.
Article188. Mège D, Figueiredo MN, Manceau G, Maggiori L, Bouhnik Y, Panis Y. Three-stage laparoscopic ileal pouch-anal anastomosis is the best approach for high-risk patients with inflammatory bowel disease: an analysis of 185 consecutive patients. J Crohns Colitis. 2016; 10:898–904.
Article189. Vogel JD, Fleshner PR, Holubar SD, et al. High complication rate after early ileostomy closure: early termination of the short versus long interval to loop ileostomy reversal after pouch surgery randomized trial. Dis Colon Rectum. 2023; 66:253–261.
Article190. Thompson DT, Hrabe JE. Staged approaches to restorative proctocolectomy with ileoanal pouch-when and why? J Laparoendosc Adv Surg Tech A. 2021; 31:875–880.
Article191. Ansaloni L, Catena F, Chattat R, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg. 2010; 97:273–280.
Article192. Oka A, Sartor RB. Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Dig Dis Sci. 2020; 65:757–788.
Article193. Mortier PE, Gambiez L, Karoui M, et al. Colectomy with ileorectal anastomosis preserves female fertility in ulcerative colitis. Gastroenterol Clin Biol. 2006; 30:594–597.
Article194. Kverneng Hultberg D, Svensson J, Jutesten H, et al. The impact of anastomotic leakage on long-term function after anterior resection for rectal cancer. Dis Colon Rectum. 2020; 63:619–628.
Article195. Uzzan M, Kirchgesner J, Oubaya N, et al. Risk of rectal neoplasia after colectomy and ileorectal anastomosis for ulcerative colitis. J Crohns Colitis. 2017; 11:930–935.
Article196. Landerholm K, Abdalla M, Myrelid P, Andersson RE. Survival of ileal pouch anal anastomosis constructed after colectomy or secondary to a previous ileorectal anastomosis in ulcerative colitis patients: a population-based cohort study. Scand J Gastroenterol. 2017; 52:531–535.
Article197. Szymańska E, Kisielewski R, Kierkuś J. Reproduction and pregnancy in inflammatory bowel disease: management and treatment based on current guidelines. J Gynecol Obstet Hum Reprod. 2021; 50:101777.198. Restellini S, Biedermann L, Hruz P, et al. Update on the management of inflammatory bowel disease during pregnancy and breastfeeding. Digestion. 2020; 101 Suppl 1:27–42.
Article199. Odufalu FD, Long M, Lin K, Mahadevan U; PIANO Investigators from the Crohn’s and Colitis Foundation (CCF) Clinical Research Alliance recruited patients for their respective centers for participant enrollment. Exposure to corticosteroids in pregnancy is associated with adverse perinatal outcomes among infants of mothers with inflammatory bowel disease: results from the PIANO registry. Gut. 2022; 71:1766–1772.200. Casanova MJ, Chaparro M, Domènech E, et al. Safety of thiopurines and anti-TNF-α drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol. 2013; 108:433–440.
Article201. Mahadevan U, Martin CF, Sandler RS, et al. PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy. Gastroenterology. 2012; 142:S-149.202. Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology. 2019; 156:1508–1524.
Article203. Mahadevan U, Robinson C, Bernasko N, et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Inflamm Bowel Dis. 2019; 25:627–641.
Article204. van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015; 9:107–124.
Article205. Nielsen OH, Gubatan JM, Juhl CB, Streett SE, Maxwell C. Biologics for inflammatory bowel disease and their safety in pregnancy: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022; 20:74–87.
Article206. Chugh R, Long MD, Jiang Y, et al. Maternal and neonatal outcomes in vedolizumab and ustekinumab exposed pregnancies: results from the PIANO registry. Am J Gastroenterol. 2024; 119:468–476.
Article207. Torres J, Chaparro M, Julsgaard M, et al. European Crohn’s and colitis guidelines on sexuality, fertility, pregnancy, and lactation. J Crohns Colitis. 2023; 17:1–27.
Article208. Akiyama S, Steinberg JM, Kobayashi M, Suzuki H, Tsuchiya K. Pregnancy and medications for inflammatory bowel disease: an updated narrative review. World J Clin Cases. 2023; 11:1730–1740.
Article209. Guariso G, Gasparetto M. Treating children with inflammatory bowel disease: current and new perspectives. World J Gastroenterol. 2017; 23:5469–5485.
Article210. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 2015; 169:1053–1060.
Article211. Hyams J, Damaraju L, Blank M, et al. Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2012; 10:391–399.
Article212. Croft NM, Faubion WA Jr, Kugathasan S, et al. Efficacy and safety of adalimumab in paediatric patients with moderate-to-severe ulcerative colitis (ENVISION I): a randomised, controlled, phase 3 study. Lancet Gastroenterol Hepatol. 2021; 6:616–627.
Article213. Hyams JS, O'Brien CD, Padgett L, et al. Maintenance golimumab treatment in pediatric UC patients with moderately to severely active UC: PURSUIT PEDS PK long-term study results. Crohns Colitis 360. 2020; 2:otaa063.
Article214. Shah P, McDonald D. Vedolizumab: an emerging treatment option for pediatric inflammatory bowel disease. J Pediatr Pharmacol Ther. 2021; 26:795–801.
Article215. Dhaliwal J, McKay HE, Deslandres C, et al. One-year outcomes with ustekinumab therapy in infliximab-refractory paediatric ulcerative colitis: a multicentre prospective study. Aliment Pharmacol Ther. 2021; 53:1300–1308.
Article216. Jerger L, Hyams JS. Special considerations for biologic medications in pediatric ulcerative colitis. Expert Opin Biol Ther. 2020; 20:429–435.
Article217. Moore H, Dubes L, Fusillo S, Baldassano R, Stein R. Tofacitinib therapy in children and young adults with pediatric-onset medically refractory inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2021; 73:e57–e62.
Article218. Nimmons D, Limdi JK. Elderly patients and inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2016; 7:51–65.
Article219. Higashiyama M, Sugita A, Koganei K, et al. Management of elderly ulcerative colitis in Japan. J Gastroenterol. 2019; 54:571–586.220. Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022; 16:2–17.
Article221. Sturm A, Maaser C, Mendall M, et al. European Crohn’s and Colitis Organisation topical review on IBD in the elderly. J Crohns Colitis. 2017; 11:263–273.222. Lin WC, Tung CC, Lin HH, et al. Elderly adults with late-onset ulcerative colitis tend to have atypical, milder initial clinical presentations but higher surgical rates and mortality: a Taiwan Society of Inflammatory Bowel Disease Study. J Am Geriatr Soc. 2016; 64:e95–e97.
Article223. Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015; 372:1441–1452.
Article224. Hsiao SW, Yen HH, Chen YY. Chemoprevention of colitis-associated dysplasia or cancer in inflammatory bowel disease. Gut Liver. 2022; 16:840–848.
Article225. Chiang CJ, Chen YC, Chen CJ, You SL, Lai MS; Taiwan Cancer Registry Task Force. Cancer trends in Taiwan. Jpn J Clin Oncol. 2010; 40:897–904.
Article226. Al Bakir I, Kabir M, Yalchin M, Hart A. Optimising inflammatory bowel disease surveillance and dysplasia management: where do we stand? United European Gastroenterol J. 2022; 10:1054–1062.
Article227. Murthy SK, Feuerstein JD, Nguyen GC, Velayos FS. AGA clinical practice update on endoscopic surveillance and management of colorectal dysplasia in inflammatory bowel diseases: expert review. Gastroenterology. 2021; 161:1043–1051.
Article228. Wijnands AM, Mahmoud R, Lutgens MW, Oldenburg B. Surveillance and management of colorectal dysplasia and cancer in inflammatory bowel disease: current practice and future perspectives. Eur J Intern Med. 2021; 93:35–41.
Article229. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018; 53:305–353.
Article230. Vienne A, Simon T, Cosnes J, et al. Low prevalence of colonoscopic surveillance of inflammatory bowel disease patients with longstanding extensive colitis: a clinical practice survey nested in the CESAME cohort. Aliment Pharmacol Ther. 2011; 34:188–195.
Article231. Gordon C, Chee D, Hamilton B, et al. Root-cause analyses of missed opportunities for the diagnosis of colorectal cancer in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2021; 53:291–301.
Article232. Kandiah K, Subramaniam S, Thayalasekaran S, et al. Multicentre randomized controlled trial on virtual chromoendoscopy in the detection of neoplasia during colitis surveillance high-definition colonoscopy (the VIRTUOSO trial). Gut. 2021; 70:1684–1690.
Article233. Adamina M, Feakins R, Iacucci M, et al. ECCO topical review optimising reporting in surgery, endoscopy, and histopathology. J Crohns Colitis. 2021; 15:1089–1105.
Article234. Hirai M, Yanai S, Kunisaki R, et al. Effectiveness of endoscopic resection for colorectal neoplasms in ulcerative colitis: a multicenter registration study. Gastrointest Endosc. 2023; 98:806–812.
Article235. Mohapatra S, Sankaramangalam K, Lopimpisuth C, et al. Advanced endoscopic resection for colorectal dysplasia in inflammatory bowel disease: a meta-analysis. Endosc Int Open. 2022; 10:E593–E601.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemoprevention of Colitis-Associated Dysplasia or Cancer in Inflammatory Bowel Disease
- Balloon-Assisted Enteroscopy and Capsule Endoscopy in Suspected Small Bowel Crohn's Disease
- Therapeutic Drug Monitoring of Biologics for Patients with Inflammatory Bowel Diseases: How, When, and for Whom?
- Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan Society of Inflammatory Bowel Disease
- Association of young age and male sex with primary sclerosing cholangitis in Taiwanese patients with inflammatory bowel disease