Cardiovasc Prev Pharmacother.
2024 Jul;6(3):85-91. 10.36011/cpp.2024.6.e11.
Re-evaluating the PCSK9 guidelines for low-density lipoprotein cholesterol targets: weighing the benefits against the risks
- Affiliations
-
- 1Lipid Clinic, Department of Cardiology, Basingstoke and North Hampshire Hospital, Hampshire Hospitals NHS Foundation Trust, Basingstoke, UK
- KMID: 2558118
- DOI: http://doi.org/10.36011/cpp.2024.6.e11
Abstract
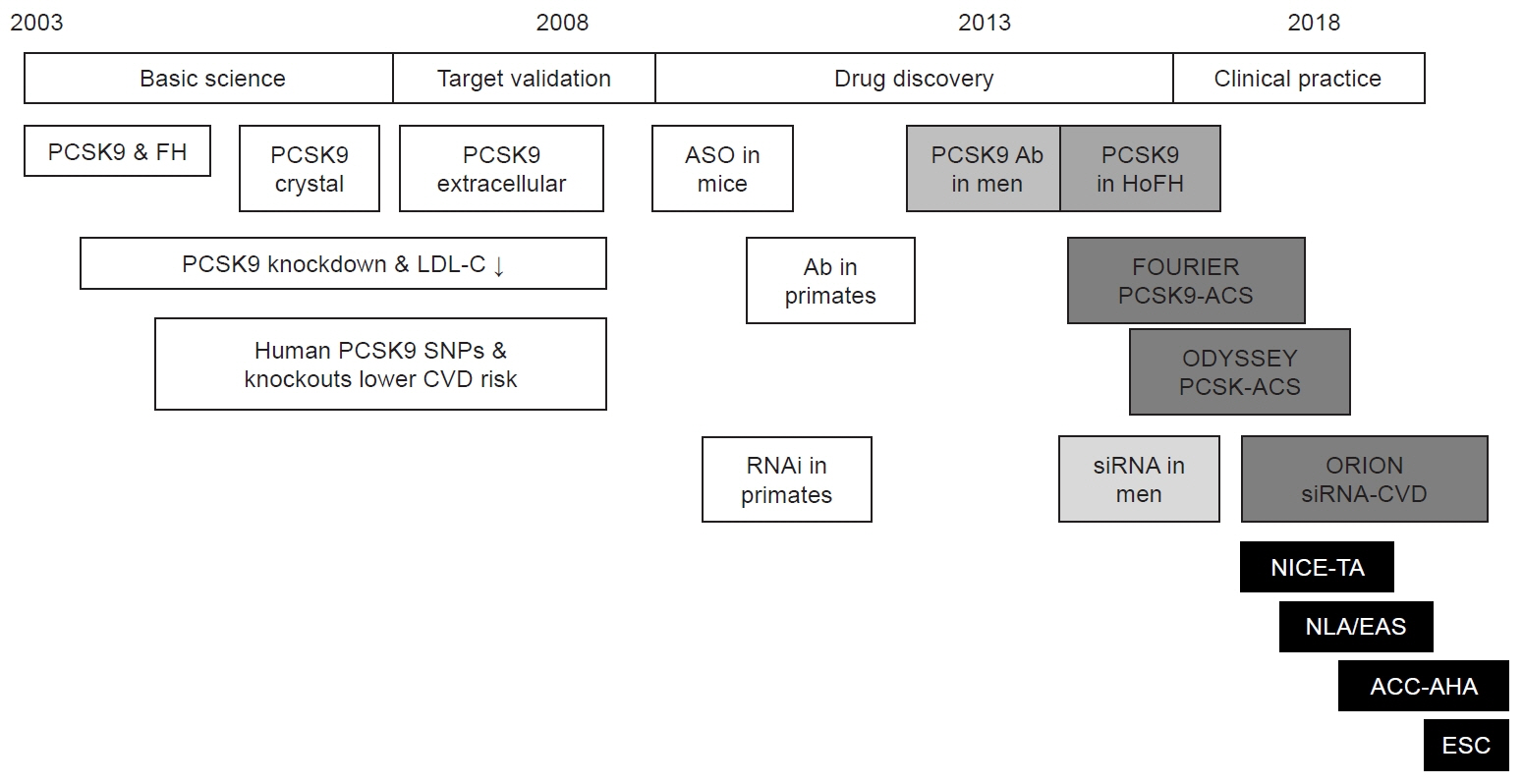
- Cardiovascular disease management has made significant progress with lipid-lowering interventions, primarily statin therapy. However, statins' side effects, combined with their variable efficacy, have sparked interest in alternative treatments, particularly proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies. These biologics, approved by the US Food and Drug Administration and the European Medicines Agency, have shown a significant impact on lipid levels, particularly low-density lipoprotein cholesterol (LDL-C), resulting in a 50% to 60% reduction. Despite the benefits of PCSK9 inhibitors, the guidelines for their use differ, with specific thresholds determining eligibility. The National Institute for Health and Care Excellence recommends starting PCSK9 therapy for patients with LDL-C levels above 3.5 mmol/L and lipid levels above 5.0 mmol/L who do not have cardiovascular disease. This rigid framework, while cost-effective, may exclude a subset of patients who do not meet these criteria despite having a high cardiovascular risk. The limited scope of these guidelines presents a challenge for specialists managing patients excluded as a result of LDL-C levels that fall just below the threshold but still show signs of significant cardiovascular risk. Recent audits revealed that a significant proportion of patients fall into this grey area, emphasizing the importance of re-evaluating LDL targets for PCSK9 inhibitor initiation. Biological variations, pharmacogenomics, and other factors all contribute to this challenge, highlighting the importance of personalized medicine.
Figure
Reference
-
1. Bitton A, Gaziano TA. The Framingham Heart Study's impact on global risk assessment. Prog Cardiovasc Dis. 2010; 53:68–78.2. Cullen P, Schulte H, Assmann G. The Münster Heart Study (PROCAM): total mortality in middle-aged men is increased at low total and LDL cholesterol concentrations in smokers but not in nonsmokers. Circulation. 1997; 96:2128–36.3. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017; 38:2459–72.4. Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part III: mechanistically defining the role of hyperlipidemia. J Lipid Res. 2005; 46:2037–51.5. Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part II. The early evidence linking hypercholesterolemia to coronary disease in humans. J Lipid Res. 2005; 46:179–90.6. British Heart Foundation. UK factsheet: our vision is a world free from the fear of heart and circulatory diseases. British Heart Foundation; 2024.7. Bradley CK, Wang TY, Li S, Robinson JG, Roger VL, Goldberg AC, et al. Patient-reported reasons for declining or discontinuing statin therapy: insights from the PALM registry. J Am Heart Assoc. 2019; 8:e011765.8. Hirsh BJ, Smilowitz NR, Rosenson RS, Fuster V, Sperling LS. Utilization of and adherence to guideline-recommended lipid-lowering therapy after acute coronary syndrome: opportunities for improvement. J Am Coll Cardiol. 2015; 66:184–92.9. Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003; 34:154–6.10. Kim EJ, Wierzbicki AS. The history of proprotein convertase subtilisin kexin-9 inhibitors and their role in the treatment of cardiovascular disease. Ther Adv Chronic Dis. 2020; 11:2040622320924569.11. National Institute for Health and Care Excellence (NICE). Alirocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia: technology appraisal guidance. NICE; 2016.12. Lin JL, Huang PH, Yeh HI, Li YH. Appropriate use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors for atherosclerotic cardiovascular disease: comparison of recommendations from different guidelines or consensus around the world. Acta Cardiol Sin. 2020; 36:403–8.13. Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020; 382:1520–30.14. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016; 37:2999–3058.15. Bolodeoku J, Gbaa T, Morris K, Whitehead S. Medicine optimisation using PCSK9 monoclonal antibodies (alirocumab and evolocumab) concerning the European Atherosclerosis Society (EAS) of < 1.8 mmol/l LDL cholesterol target in a secondary care lipid clinic. Br J Healthc Med Res. 2023; 10:91–102.16. Carr DF, Francis B, Jorgensen AL, Zhang E, Chinoy H, Heckbert SR, et al. Genomewide association study of statin-induced myopathy in patients recruited using the UK clinical practice research datalink. Clin Pharmacol Ther. 2019; 106:1353–61.17. Hayat M, Kerr R, Bentley AR, Rotimi CN, Raal FJ, Ramsay M. Correction: genetic associations between serum low LDL-cholesterol levels and variants in LDLR, APOB, PCSK9 and LDLRAP1 in African populations. PLoS One. 2021; 16:e0249478.18. Nguyen HT, Ha KP, Nguyen AH, Nguyen TT, Lam HM. Non-achievement of the low-density lipoprotein cholesterol goal in older patients with type 2 diabetes mellitus and a very high cardiovascular disease risk: a multicenter study in Vietnam. Ann Geriatr Med Res. 2021; 25:278–85.19. Kim S, Han S, Rane PP, Qian Y, Zhao Z, Suh HS. Achievement of the low-density lipoprotein cholesterol goal among patients with dyslipidemia in South Korea. PLoS One. 2020; 15:e0228472.20. Ray KK, Raal FJ, Kallend DG, Jaros MJ, Koenig W, Leiter LA, et al. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. 2023; 44:129–38.21. Suresh A, Theodoraki A, Ward E, Feher M. PCSK9 inhibitors and treatment targets: an audit-based evaluation of a specialist lipid clinic. Br J Diabetes. 2022; 22:30–35.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Drugs for Treating Dyslipidemia: Beyond Statins
- Lipid-Lowering Therapy Guidelines
- New Therapeutic Approaches to the Treatment of Dyslipidemia 2: LDL-C and Lp(a)
- Pharmacological Strategies beyond Statins: Ezetimibe and PCSK9 Inhibitors
- Detection of Familial Hypercholesterolemia Using Next Generation Sequencing in Two Population-Based Cohorts