Ann Lab Med.
2024 Jul;44(4):314-323. 10.3343/alm.2023.0382.
Manufacturing Cell and Gene Therapies: Challenges in Clinical Translation
- Affiliations
-
- 1Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences and Technology (SAIHST), Sungkyunkwan University, Seoul, Korea
- 2Cell and Gene Therapy Institute (CGTI), Research Institute for Future Medicine, Samsung Medical Center, Seoul, Korea; 3 Cell and Gene Therapy Institute, ENCell Co. Ltd., Seoul, Korea
- KMID: 2557930
- DOI: http://doi.org/10.3343/alm.2023.0382
Abstract
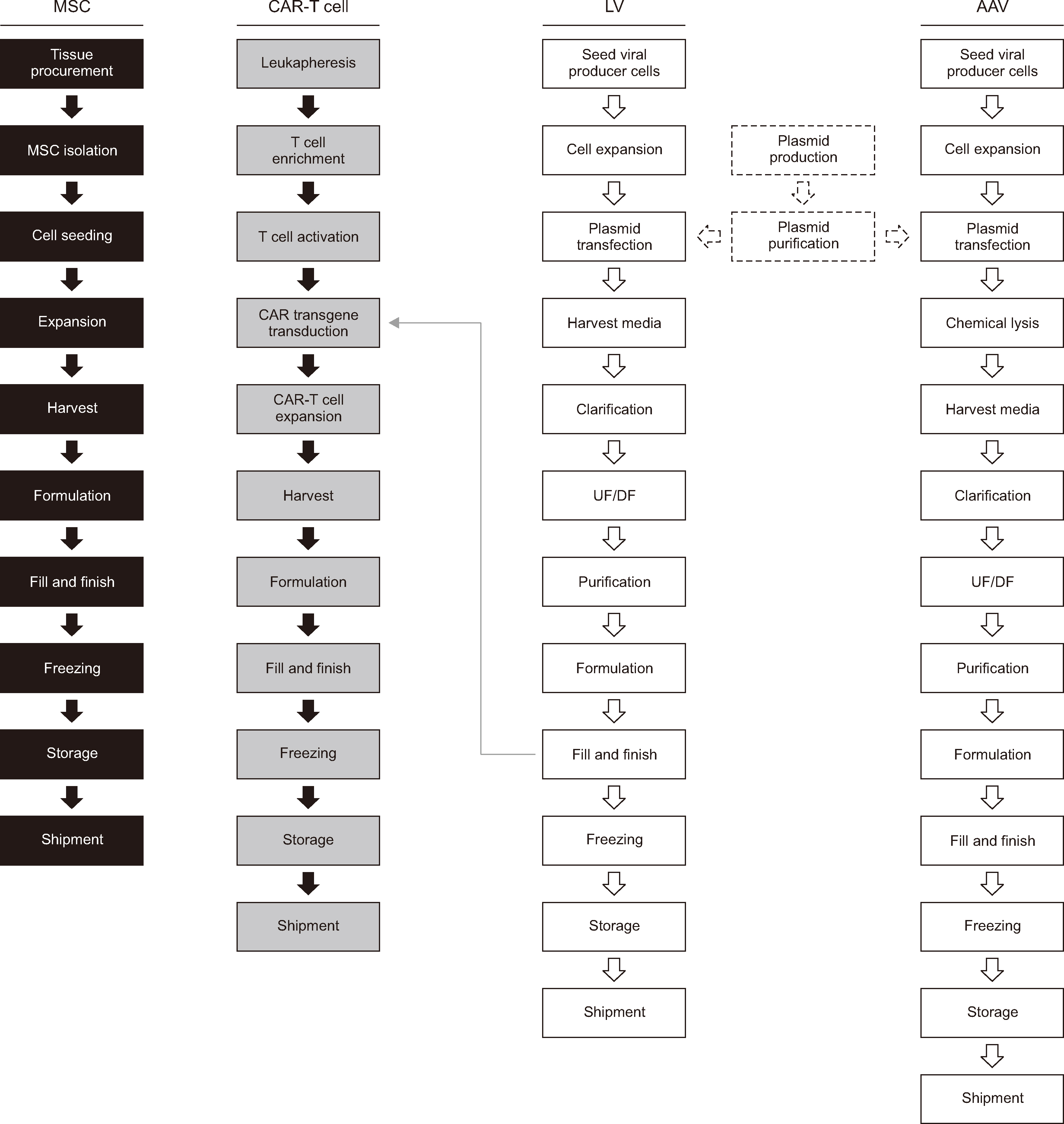
- The safety and efficacy of both cell and gene therapies have been demonstrated in numerous preclinical and clinical trials. Chimeric antigen receptor T (CAR-T) cell therapy, which leverages the technologies of both cell and gene therapies, has also shown great promise for treating various cancers. Advancements in pertinent fields have also highlighted challenges faced while manufacturing cell and gene therapy products. Potential problems and obstacles must be addressed to ease the clinical translation of individual therapies. Literature reviews of representative cell-based, gene-based, and cell-based gene therapies with regard to their general manufacturing processes, the challenges faced during manufacturing, and QC specifications are limited. We review the general manufacturing processes of cell and gene therapies, including those involving mesenchymal stem cells, viral vectors, and CAR-T cells. The complexities associated with the manufacturing processes and subsequent QC/validation processes may present challenges that could impede the clinical progression of the products. This article addresses these potential challenges. Further, we discuss the use of the manufacturing model and its impact on cell and gene therapy.
Keyword
Figure
Reference
-
References
1. Arabi F, Mansouri V, Ahmadbeigi N. 2022; Gene therapy clinical trials, where do we go? An overview. Biomed Pharmacother. 153:113324. DOI: 10.1016/j.biopha.2022.113324. PMID: 35779421.
Article2. Sanz-Nogués C, O'Brien T. 2021; Current good manufacturing practice considerations for mesenchymal stromal cells as therapeutic agents. Biomater Biosyst. 2:100018. DOI: 10.1016/j.bbiosy.2021.100018. PMID: 36824657. PMCID: PMC9934414.
Article3. Papaioannou I, Owen JS, Yáñez-Muñoz RJ. 2023; Clinical applications of gene therapy for rare diseases: a review. Int J Exp Pathol. 104:154–76. DOI: 10.1111/iep.12478. PMID: 37177842. PMCID: PMC10349259.
Article4. Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V. 2020; Therapeutic potential of mesenchymal stem cells for cancer therapy. Front Bioeng Biotechnol. 8:43. DOI: 10.3389/fbioe.2020.00043. PMID: 32117924. PMCID: PMC7013101. PMID: f017bcafff6042bc80af1d5d1aae7c5d.
Article5. Heathman TR, Nienow AW, McCall MJ, Coopman K, Kara B, Hewitt CJ. 2015; The translation of cell-based therapies: clinical landscape and manufacturing challenges. Regen Med. 10:49–64. DOI: 10.2217/rme.14.73. PMID: 25562352.
Article6. Kim I. 2013; A brief overview of cell therapy and its product. J Korean Assoc Oral Maxillofac Surg. 39:201–2. DOI: 10.5125/jkaoms.2013.39.5.201. PMID: 24471045. PMCID: PMC3858137.
Article7. El-Kadiry AE, Rafei M, Shammaa R. 2021; Cell therapy: types, regulation, and clinical benefits. Front Med (Lausanne). 8:756029. DOI: 10.3389/fmed.2021.756029. PMID: 34881261. PMCID: PMC8645794. PMID: 4b199b82483b4804886346411fff7906.
Article8. Moody J, Milligan WD, St Onge M, Goonewardene A, Rivers P. 2021; Cell and gene therapy: a snapshot of investor perspectives. Cytotherapy. 23:256–60. DOI: 10.1016/j.jcyt.2020.11.005. PMID: 33281066.
Article9. Wang LL, Janes ME, Kumbhojkar N, Kapate N, Clegg JR, Prakash S, et al. 2021; Cell therapies in the clinic. Bioeng Transl Med. 6:e10214. DOI: 10.1002/btm2.10214. PMID: 34027097. PMCID: PMC8126820. PMID: 579dc17147a447c4878ce11b58f3e9cb.
Article10. Schumann GG, Fuchs NV, Tristán-Ramos P, Sebe A, Ivics Z, Heras SR. 2019; The impact of transposable element activity on therapeutically relevant human stem cells. Mob DNA. 10:9. DOI: 10.1186/s13100-019-0151-x. PMID: 30899334. PMCID: PMC6408843. PMID: 754709407bfe48578aa76e7b6d842beb.
Article11. Li Y, Hao J, Hu Z, Yang YG, Zhou Q, Sun L, et al. 2022; Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: a systematic review. Stem Cell Res Ther. 13:93. DOI: 10.1186/s13287-022-02751-0. PMID: 35246235. PMCID: PMC8895864. PMID: a55056317223417d8d35a4a7773ee2ec.
Article12. Fernández-Santos ME, Garcia-Arranz M, Andreu EJ, García-Hernández AM, López-Parra M, Villarón E, et al. 2022; Optimization of mesenchymal stromal cell (MSC) manufacturing processes for a better therapeutic outcome. Front Immunol. 13:918565. DOI: 10.3389/fimmu.2022.918565. PMID: 35812460. PMCID: PMC9261977. PMID: 859113e2a67f49de8a632985ba59dbc7.
Article13. Costela-Ruiz VJ, Melguizo-Rodríguez L, Bellotti C, Illescas-Montes R, Stanco D, Arciola CR, et al. 2022; Different sources of mesenchymal stem cells for tissue regeneration: a guide to identifying the most favorable one in orthopedics and dentistry applications. Int J Mol Sci. 23:6356. DOI: 10.3390/ijms23116356. PMID: 35683035. PMCID: PMC9181542. PMID: 778d4cd136e743f0ab982af16e2e914c.
Article14. Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. 2018; Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 93:19–31. DOI: 10.1002/cyto.a.23242. PMID: 29072818.
Article15. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. 2019; Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 4:22. DOI: 10.1038/s41536-019-0083-6. PMID: 31815001. PMCID: PMC6889290.
Article16. Kusuma GD, Carthew J, Lim R, Frith JE. 2017; Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 26:617–31. DOI: 10.1089/scd.2016.0349. PMID: 28186467.
Article17. Jossen V, van den Bos C, Eibl R, Eibl D. 2018; Manufacturing human mesenchymal stem cells at clinical scale: process and regulatory challenges. Appl Microbiol Biotechnol. 102:3981–94. DOI: 10.1007/s00253-018-8912-x. PMID: 29564526. PMCID: PMC5895685.
Article18. Hassan MNFB, Yazid MD, Yunus MHM, Chowdhury SR, Lokanathan Y, Idrus RBH, et al. 2020; Large-scale expansion of human mesenchymal stem cells. Stem Cells Int. 2020:9529465. DOI: 10.1155/2020/9529465. PMID: 32733574. PMCID: PMC7378617. PMID: 76b3f189901d4e48bcc7597e660650ef.
Article19. Guadix JA, López-Beas J, Clares B, Soriano-Ruiz JL, Zugaza JL, Gálvez-Martín P. 2019; Principal criteria for evaluating the quality, safety and efficacy of hMSC-based products in clinical practice: current approaches and challenges. Pharmaceutics. 11:552. DOI: 10.3390/pharmaceutics11110552. PMID: 31652984. PMCID: PMC6921040. PMID: 42c29149602241f084188c2e66b1a87b.
Article20. Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, et al. 2021; Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 14:24. DOI: 10.1186/s13045-021-01037-x. PMID: 33579329. PMCID: PMC7880217. PMID: 84bd903655854a26891e4357d80ddf37.
Article21. Zhao Z, Anselmo AC, Mitragotri S. 2022; Viral vector-based gene therapies in the clinic. Bioeng Transl Med. 7:e10258. DOI: 10.1002/btm2.10258. PMID: 35079633. PMCID: PMC8780015. PMID: 862afd99f3c44e21b81c77ad10af25b0.22. Kang L, Jin S, Wang J, Lv Z, Xin C, Tan C, et al. 2023; AAV vectors applied to the treatment of CNS disorders: clinical status and challenges. J Control Release. 355:458–73. DOI: 10.1016/j.jconrel.2023.01.067. PMID: 36736907.
Article23. Kingwell K. 2022; Lentiviral vector gene therapies come of age with two FDA approvals. Nat Rev Drug Discov. 21:790–1. DOI: 10.1038/d41573-022-00176-1. PMID: 36241845.
Article24. van der Walle CF, Dufès C, Desai AS, Kerby J, Broadhead J, Tam A, et al. 2022; Report on webinar series cell and gene therapy: from concept to clinical use. Pharmaceutics. 14:168. DOI: 10.3390/pharmaceutics14010168. PMID: 35057063. PMCID: PMC8778748. PMID: 9bd39ba2c93c48cab4aae13fdbdd5c9c.
Article25. Munis AM. 2020; Gene therapy applications of non-human lentiviral vectors. Viruses. 12:1106. DOI: 10.3390/v12101106. PMID: 33003635. PMCID: PMC7599719. PMID: 7d8f816a82164ee08495b330db61dba9.
Article26. Sabatino DE, Bushman FD, Chandler RJ, Crystal RG, Davidson BL, Dolmetsch R, et al. 2022; Evaluating the state of the science for adeno-associated virus integration: an integrated perspective. Mol Ther. 30:2646–63. DOI: 10.1016/j.ymthe.2022.06.004. PMID: 35690906. PMCID: PMC9372310.
Article27. Bisserier M, Sun XQ, Fazal S, Turnbull IC, Bonnet S, Hadri L. 2022; Novel insights into the therapeutic potential of lung-targeted gene transfer in the most common respiratory diseases. Cells. 11:984. DOI: 10.3390/cells11060984. PMID: 35326434. PMCID: PMC8947048. PMID: d8fda3586b1a42f8b1182e6fa9c274bb.
Article28. Srivastava A, Mallela KMG, Deorkar N, Brophy G. 2021; Manufacturing challenges and rational formulation development for AAV viral vectors. J Pharm Sci. 110:2609–24. DOI: 10.1016/j.xphs.2021.03.024. PMID: 33812887.
Article29. Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. 2021; Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. 6:53. DOI: 10.1038/s41392-021-00487-6. PMID: 33558455. PMCID: PMC7868676. PMID: 63666028eb554ff786d8586456529231.
Article30. Wright JF. 2021; Quality control testing, characterization and critical quality attributes of adeno-associated virus vectors used for human gene therapy. Biotechnol J. 16:e2000022. DOI: 10.1002/biot.202000022. PMID: 33146911.
Article31. Shi R, Jia S, Liu H, Nie H. 2022; Clinical grade lentiviral vector purification and quality control requirements. J Sep Sci. 45:2093–101. DOI: 10.1002/jssc.202100937. PMID: 35247228.
Article32. Labbé RP, Vessillier S, Rafiq QA. 2021; Lentiviral vectors for T cell engineering: clinical applications, bioprocessing and future perspectives. Viruses. 13:1528. DOI: 10.3390/v13081528. PMID: 34452392. PMCID: PMC8402758. PMID: 2d62769bd9554d35a58221ab3996c089.
Article33. Perry C, Rayat ACME. 2021; Lentiviral vector bioprocessing. Viruses. 13:268. DOI: 10.3390/v13020268. PMID: 33572347. PMCID: PMC7916122. PMID: db12b1bd63064381b315ae5a169c013a.
Article34. Croissant C, Armitano J, Lazuech B, Švec D, Pugin C, Guesdon A, et al. 2023; A new T-antigen negative HEK293 cell line with improved AAV productivity. Biotechnol Bioeng. 120:1953–60. DOI: 10.1002/bit.28414. PMID: 37232541.
Article35. Ferreira MV, Cabral ET, Coroadinha AS. 2021; Progress and perspectives in the development of lentiviral vector producer cells. Biotechnol J. 16:e2000017. DOI: 10.1002/biot.202000017. PMID: 32686901.
Article36. Yuan Z, Qiao C, Hu P, Li J, Xiao X. 2011; A versatile adeno-associated virus vector producer cell line method for scalable vector production of different serotypes. Hum Gene Ther. 22:613–24. DOI: 10.1089/hum.2010.241. PMID: 21186998. PMCID: PMC3081441.
Article37. Selvaraj N, Wang CK, Bowser B, Broadt T, Shaban S, Burns J, et al. 2021; Detailed protocol for the novel and scalable viral vector upstream process for AAV gene therapy manufacturing. Hum Gene Ther. 32:850–61. DOI: 10.1089/hum.2020.054. PMID: 33397196. PMCID: PMC8418526.
Article38. Chen YH, Pallant C, Sampson CJ, Boiti A, Johnson S, Brazauskas P, et al. 2020; Rapid lentiviral vector producer cell line generation using a single DNA construct. Mol Ther Methods Clin Dev. 19:47–57. DOI: 10.1016/j.omtm.2020.08.011. PMID: 32995359. PMCID: PMC7501408.
Article39. Wada M, Uchida N, Posadas-Herrera G, Hayashita-Kinoh H, Tsunekawa Y, Hirai Y, et al. 2023; Large-scale purification of functional AAV particles packaging the full genome using short-term ultracentrifugation with a zonal rotor. Gene Ther. 30:641–8. DOI: 10.1038/s41434-023-00398-x. PMID: 36977769. PMCID: PMC10457186.
Article40. Pei X, Earley LF, He Y, Chen X, Hall NE, Samulski RJ, et al. 2018; Efficient capsid antigen presentation from adeno-associated virus empty virions in vivo. Front Immunol. 9:844. DOI: 10.3389/fimmu.2018.00844. PMID: 29725339. PMCID: PMC5916967. PMID: de5b08b5f607400c82643773f48e5af6.41. Fu Q, Polanco A, Lee YS, Yoon S. 2023; Critical challenges and advances in recombinant adeno-associated virus (rAAV) biomanufacturing. Biotechnol Bioeng. 120:2601–21. DOI: 10.1002/bit.28412. PMID: 37126355.
Article42. Timmins LM, Patel RS, Teryek MS, Parekkadan B. 2019; Real-time transfer of lentiviral particles by producer cells using an engineered coculture system. Cytotechnology. 71:1019–31. DOI: 10.1007/s10616-019-00343-0. PMID: 31515650. PMCID: PMC6787137.
Article43. Tran MY, Kamen AA. 2022; Production of lentiviral vectors using a HEK-293 producer cell line and advanced perfusion processing. Front Bioeng Biotechnol. 10:887716. DOI: 10.3389/fbioe.2022.887716. PMID: 35774066. PMCID: PMC9237754. PMID: 8dff0a1f979149dda7991e6c635cdb6b.
Article44. Roex G, Campillo-Davo D, Flumens D, Shaw PAG, Krekelbergh L, De Reu H, et al. 2022; Two for one: targeting BCMA and CD19 in B-cell malignancies with off-the-shelf dual-CAR NK-92 cells. J Transl Med. 20:124. DOI: 10.1186/s12967-022-03326-6. PMID: 35287669. PMCID: PMC8919645. PMID: 05c1c30b2722445782796239a7b077f8.
Article45. Wei J, Han X, Bo J, Han W. 2019; Target selection for CAR-T therapy. J Hematol Oncol. 12:62. DOI: 10.1186/s13045-019-0758-x. PMID: 31221182. PMCID: PMC6587237. PMID: 8f487526a3864704af296aaea874cc21.
Article46. Mougiakakos D, Krönke G, Völkl S, Kretschmann S, Aigner M, Kharboutli S, et al. 2021; CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. 385:567–9. DOI: 10.1056/NEJMc2107725. PMID: 34347960.
Article47. Lee HJ, Kim SK, Cho D, Lee JJ. 2015; Cellular immunotherapy as a beacon of hope for hematological malignancies. Blood Res. 50:126–8. DOI: 10.5045/br.2015.50.3.126. PMID: 26457276. PMCID: PMC4595575.
Article48. Vormittag P, Gunn R, Ghorashian S, Veraitch FS. 2018; A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol. 53:164–81. DOI: 10.1016/j.copbio.2018.01.025. PMID: 29462761.
Article49. Lv Z, Luo F, Chu Y. 2023; Strategies for overcoming bottlenecks in allogeneic CAR-T cell therapy. Front Immunol. 14:1199145. DOI: 10.3389/fimmu.2023.1199145. PMID: 37554322. PMCID: PMC10405079. PMID: 3b821db9babb429b95c2839304624d3a.
Article50. Milone MC, O'Doherty U. 2018; Clinical use of lentiviral vectors. Leukemia. 32:1529–41. DOI: 10.1038/s41375-018-0106-0. PMID: 29654266. PMCID: PMC6035154.
Article51. Medvec AR, Ecker C, Kong H, Winters EA, Glover J, Varela-Rohena A, et al. 2018; Improved expansion and in vivo function of patient T cells by a serum-free medium. Mol Ther Methods Clin Dev. 8:65–74. DOI: 10.1016/j.omtm.2017.11.001. PMID: 29687031. PMCID: PMC5907749. PMID: 4b72adeeb7ef495f864b298b3073f32a.
Article52. Koh SK, Park J, Kim SE, Lim Y, Phan MT, Kim J, et al. 2022; Natural killer cell expansion and cytotoxicity differ depending on the culture medium used. Ann Lab Med. 42:638–49. DOI: 10.3343/alm.2022.42.6.638. PMID: 35765872. PMCID: PMC9277036.
Article53. Schmidts A, Marsh LC, Srivastava AA, Bouffard AA, Boroughs AC, Scarfò I, et al. 2020; Cell-based artificial APC resistant to lentiviral transduction for efficient generation of CAR-T cells from various cell sources. J Immunother Cancer. 8:e000990. DOI: 10.1136/jitc-2020-000990. PMID: 32900862. PMCID: PMC7477986. PMID: 1383b25b9a8e4f49a0640c17e9556951.
Article54. Mikhael J, Fowler J, Shah N. 2022; Chimeric antigen receptor T-cell therapies: barriers and solutions to access. JCO Oncol Pract. 18:800–7. DOI: 10.1200/OP.22.00315. PMID: 36130152.
Article55. Jackson Z, Roe A, Sharma AA, Lopes FBTP, Talla A, Kleinsorge-Block S, et al. 2020; Automated manufacture of autologous CD19 CAR-T cells for treatment of non-Hodgkin lymphoma. Front Immunol. 11:1941. DOI: 10.3389/fimmu.2020.01941. PMID: 32849651. PMCID: PMC7427107. PMID: 8d0bad17627d4c10b8f6a1d433064624.
Article56. Gajra A, Zalenski A, Sannareddy A, Jeune-Smith Y, Kapinos K, Kansagra A. 2022; Barriers to chimeric antigen receptor T-cell (CAR-T) therapies in clinical practice. Pharmaceut Med. 36:163–71. DOI: 10.1007/s40290-022-00428-w. PMID: 35672571. PMCID: PMC9217916.
Article57. Wang X, Rivière I. 2016; Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 3:16015. DOI: 10.1038/mto.2016.15. PMID: 27347557. PMCID: PMC4909095. PMID: 5311327946444c02a2cb7b3e9317382a.
Article58. Li Y, Huo Y, Yu L, Wang J. 2019; Quality control and nonclinical research on CAR-T cell products: general principles and key issues. Engineering. 5:122–31. DOI: 10.1016/j.eng.2018.12.003.
Article59. Allen JM, Debelak DJ, Reynolds TC, Miller AD. 1997; Identification and elimination of replication-competent adeno-associated virus (AAV) that can arise by nonhomologous recombination during AAV vector production. J Virol. 71:6816–22. DOI: 10.1128/jvi.71.9.6816-6822.1997. PMID: 9261406. PMCID: PMC191962.
Article60. Food and Drug Administration. Summary basis for regulatory action. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/August-30--2017-Summary-Basis-for-Regulatory-Action---KYMRIAH.pdf. Updated on Aug 2017.61. Food and Drug Administration. Summary basis for regulatory action. https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/October-18--2017-Summary-Basis-for-Regulatory-Action---YESCARTA.pdf. Updated on Oct 2017.62. Food and Drug Administration. Summary basis for regulatory action. https://www.fda.gov/media/141093/download#:~:text=TECARTUS%27%20mechanism%20of%20action%20is,tumor%20toxicity%20from%20CD19%20targeting. Updated on July 2020.63. Food and Drug Administration. Summary basis for regulatory action. https://www.fda.gov/media/156999/download. Updated on Feb 2022.64. U.S. Food and Drug Administration. Considerations for the development of chimeric antigen receptor (CAR) T cell products: draft guidance for industry. https://www.fda.gov/media/156896/download. Updated on Jan 2024.65. Harrison RP, Rafiq QA, Medcalf N. 2018; Centralised versus decentralised manufacturing and the delivery of healthcare products: a United Kingdom exemplar. Cytotherapy. 20:873–90. DOI: 10.1016/j.jcyt.2018.05.003. PMID: 29807726.
Article66. Harrison RP, Ruck S, Medcalf N, Rafiq QA. 2017; Decentralized manufacturing of cell and gene therapies: overcoming challenges and identifying opportunities. Cytotherapy. 19:1140–51. DOI: 10.1016/j.jcyt.2017.07.005. PMID: 28797612.
Article67. Stroncek D, Dinh A, Rai H, Zhang N, Somerville R, Panch S. 2022; The need for uniform and coordinated practices involving centrally manufactured cell therapies. J Transl Med. 20:184. DOI: 10.1186/s12967-022-03385-9. PMID: 35468789. PMCID: PMC9036766. PMID: f2315bae65fe42d99051ff1f9bd99e59.
Article68. Iancu EM, Kandalaft LE. 2020; Challenges and advantages of cell therapy manufacturing under good manufacturing practices within the hospital setting. Curr Opin Biotechnol. 65:233–41. DOI: 10.1016/j.copbio.2020.05.005. PMID: 32663771.
Article69. Locke FL, Hu ZH, Siddiqi T, Jacobson CA, Nikiforow S, Ahmed S, et al. 2022; Real-world impact of time from leukapheresis to infusion (vein-to-vein time) in patients with relapsed or refractory (r/r) large B-cell lymphoma (LBCL) treated with axicabtagene ciloleucel. Blood. 140(S1):7512–5. DOI: 10.1182/blood-2022-155603.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vitro stem cell differentiation for salivary gland regeneration: a comprehensive review
- Challenges in the Use of Targeted Therapies in Non–Small Cell Lung Cancer
- Quality assessment of cellular therapies: the emerging role of molecular assays
- Strategies for Manipulating T Cells in Cancer Immunotherapy
- Advances in laser and stem cell treatment: current technologies, limitations, and future prospects