J Korean Med Sci.
2024 Jul;39(26):e220. 10.3346/jkms.2024.39.e220.
A Causality Assessment Framework for COVID-19 Vaccines and Adverse Events at the COVID-19 Vaccine Safety Research Center
- Affiliations
-
- 1COVID-19 Vaccine Safety Research Center, Seoul, Korea
- 2Department of Public Health Science, Graduate School of Public Health, Seoul National University, Seoul, Korea
- 3Department of Health Convergence, College of Science and Industry Convergence, Ewha Womans University, Seoul, Korea
- 4Department of Preventive Medicine, College of Medicine, Graduate Program in System Health Science and Engineering, Ewha Womans University, Seoul, Korea
- 5Department of Health Informatics and Biostatistics, Graduate School of Public Health, Yonsei University, Seoul, Korea
- 6Department of Psychiatry, Uijeongbu Eulji Medical Center, Eulji University School of Medicine, Uijeongbu, Korea
- 7Department of Radiology, Eunpyeong St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- 8Department of Infectious Diseases, Daejeon Eulji Medical Center, Eulji University School of Medicine, Daejeon, Korea
- 9National Cancer Center, Goyang, Korea
- 10Department of Physiology, College of Medicine and Neuroscience Research Institute, Korea University, Seoul, Korea
- 11Department of Medicine, Korea Tourism College Eldercare Center, Icheon, Korea
- 12National Academy of Medicine of Korea, Seoul, Korea
- 13Department of Social and Preventive Medicine, Hallym University College of Medicine, Chuncheon, Korea
- KMID: 2557778
- DOI: http://doi.org/10.3346/jkms.2024.39.e220
Abstract
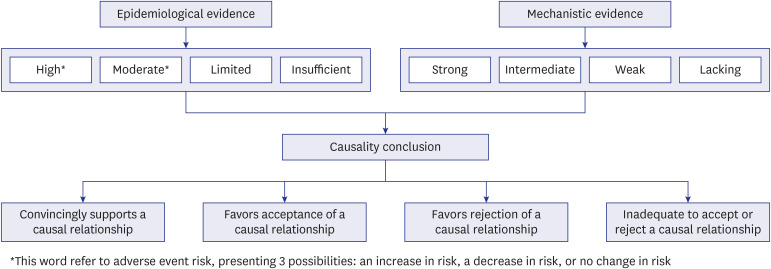
- During the coronavirus disease 2019 (COVID-19) pandemic, conclusively evaluating possible associations between COVID-19 vaccines and potential adverse events was of critical importance. The National Academy of Medicine of Korea established the COVID-19 Vaccine Safety Research Center (CoVaSC) with support from the Korea Disease Control and Prevention Agency to investigate the scientific relationship between COVID-19 vaccines and suspected adverse events. Although determining whether the COVID-19 vaccine was responsible for any suspected adverse event necessitated a systematic approach, traditional causal inference theories, such as Hill's criteria, encountered certain limitations and criticisms. To facilitate a systematic and evidence-based evaluation, the United States Institute of Medicine, at the request of the Centers for Disease Control and Prevention, offered a detailed causality assessment framework in 2012, which was updated in the recent report by the National Academies of Sciences, Engineering, and Medicine (NASEM) in 2024. This framework, based on a weight-of-evidence approach, allows the independent evaluation of both epidemiological and mechanistic evidence, culminating in a comprehensive conclusion about causality. Epidemiological evidence derived from population studies is categorized into four levels—high, moderate, limited, or insufficient—while mechanistic evidence, primarily from biological and clinical studies in animals and individuals, is classified as strong, intermediate, weak, or lacking. The committee then synthesizes these two types of evidence to draw a conclusion about the causal relationship, which can be described as “convincingly supports” (“evidence established” in the 2024 NASEM report), “favors acceptance,” “favors rejection,” or “inadequate to accept or reject.” The CoVaSC has established an independent committee to conduct causality assessments using the weightof-evidence framework, specifically for evaluating the causality of adverse events associated with COVID-19 vaccines. The aim of this study is to provide an overview of the weight-ofevidence framework and to detail the considerations involved in its practical application in the CoVaSC.
Keyword
Figure
Reference
-
1. Jeong NY, Park H, Oh S, Jung SE, Kim DH, Shin HS, et al. A framework for nationwide COVID-19 vaccine safety research in the Republic of Korea: the COVID-19 Vaccine Safety Research Committee. Osong Public Health Res Perspect. 2023; 14(1):5–14. PMID: 36944340.2. Maldonado G, Cox LA. Causal reasoning in epidemiology: philosophy and logic. Glob Epidemiol. 2020; 2:100020.3. Institute of Medicine. Adverse Effects of Vaccines: Evidence and Causality. Washington, D.C., USA: The National Academies Press;2012.4. National Academies of Sciences, Engineering, and Medicine. Evidence Review of the Adverse Effects of COVID-19 Vaccination and Intramuscular Vaccine Administration. Washington, D.C.: National Academies of Sciences, Engineering, and Medicine;2024.5. United States Surgeon General’s Advisory Committee on Smoking. Summary of the Report of the Surgeon General’s Advisory Committee on Smoking and Health. Washington, D.C., USA: US Department of Health, Education, and Welfare, Public Health Service;1964.6. Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965; 58(5):295–300. PMID: 14283879.7. Howick J, Glasziou P, Aronson JK. The evolution of evidence hierarchies: what can Bradford Hill’s ‘guidelines for causation’ contribute? J R Soc Med. 2009; 102(5):186–194. PMID: 19417051.8. World Health Organization. Causality Assessment of an Adverse Event Following Immunization (AEFI): User Manual for the Revised WHO Classification Second Edition, 2019 Update. Geneva, Switzerland: World Health Organization;2019.9. MacIntyre CR. Using the Bradford-Hill criteria to assess causality in the association between CHADOX1 NCOV-19 vaccine and prothrombotic immune thrombocytopenia. Glob Biosecur. 2021; 3(1):10. Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia, PA, USA: Lippincott-Raven Publishers;1998.11. Hong KS, Park BJ, Shin SG, Yang JS, Lee SM, Kim Y, et al. Development of Korean algorithm for causality assessment of adverse drug reactions. J Korean Soc Clin Pharmacol Ther. 2002; 10(2):129–142.12. Kim BR, Lee JS, Han OY, Jeong SH, Yim HW, Jung KE, et al. Comparison between WHO-UMC causality categories and Korean algorithm version 2.0 for causality evaluation of adverse drug reactions. J Pharmacoepidemiol Risk Manag. 2012; 5(1):31–39.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COVID-19 vaccine safety monitoring in the Republic of Korea: February 26, 2021 to April 30, 2021
- Post-vaccination COVID-19 deaths: a review of available evidence and recommendations for the global population
- COVID-19 Vaccination for Pilots and Air Traffic Controllers
- Adverse events and preventive measures related to COVID-19 vaccines
- COVID-19 vaccine safety monitoring in Republic of Korea from February 26, 2021 to October 31, 2021