J Pathol Transl Med.
2024 Jul;58(4):191-197. 10.4132/jptm.2024.05.14.
Concurrent intestinal plasmablastic lymphoma and diffuse large B-cell lymphoma with a clonal relationship: a case report and literature review
- Affiliations
-
- 1Medical Student, National Defense Medical College, Tokorozawa, Japan
- 2Department of Pathology and Laboratory Medicine, National Defense Medical College, Tokorozawa, Japan
- 3Department of Laboratory Medicine, National Defense Medical College Hospital, Tokorozawa, Japan
- 4Department of Hematology, National Defense Medical College, Tokorozawa, Japan
- KMID: 2557759
- DOI: http://doi.org/10.4132/jptm.2024.05.14
Abstract
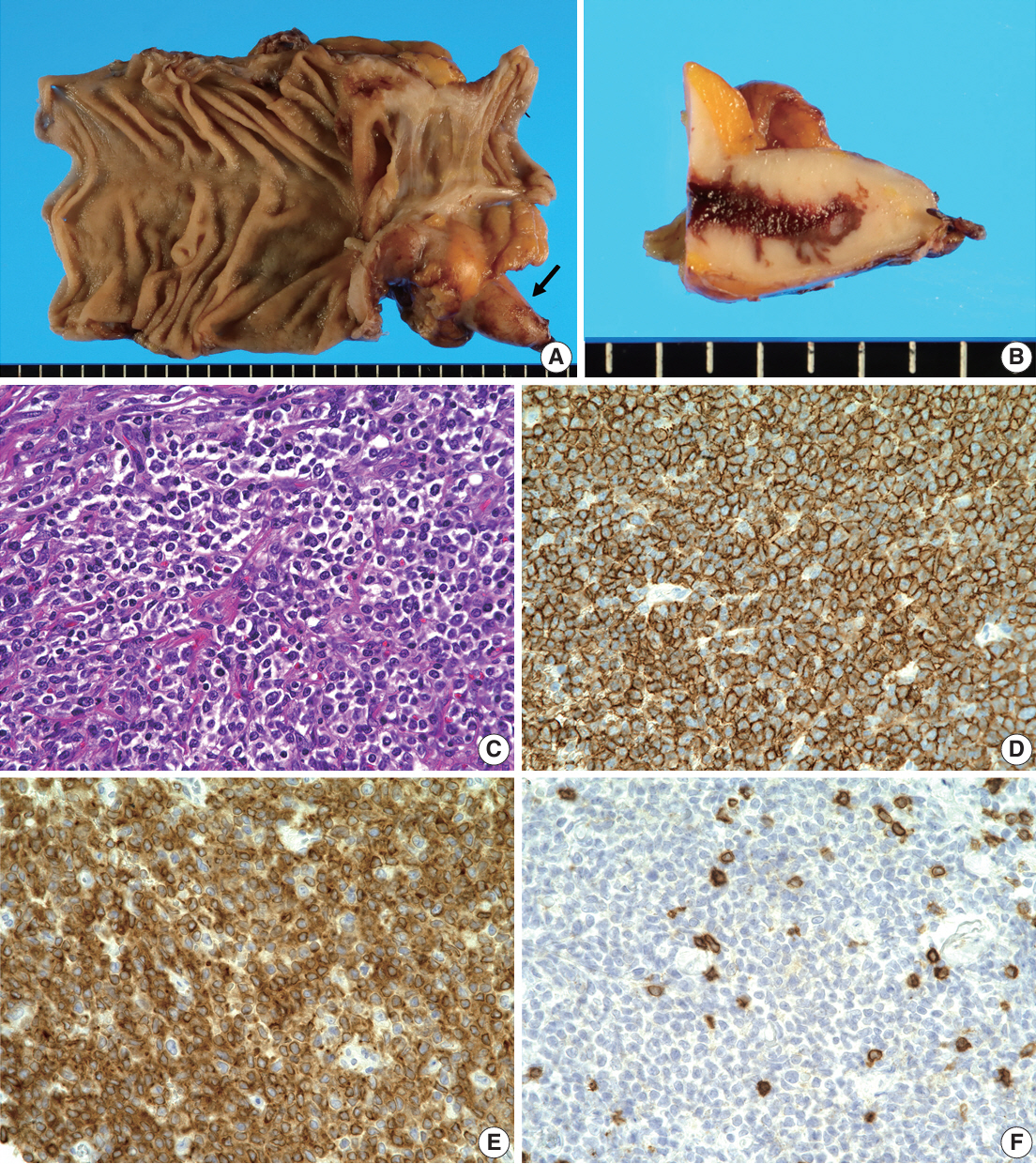
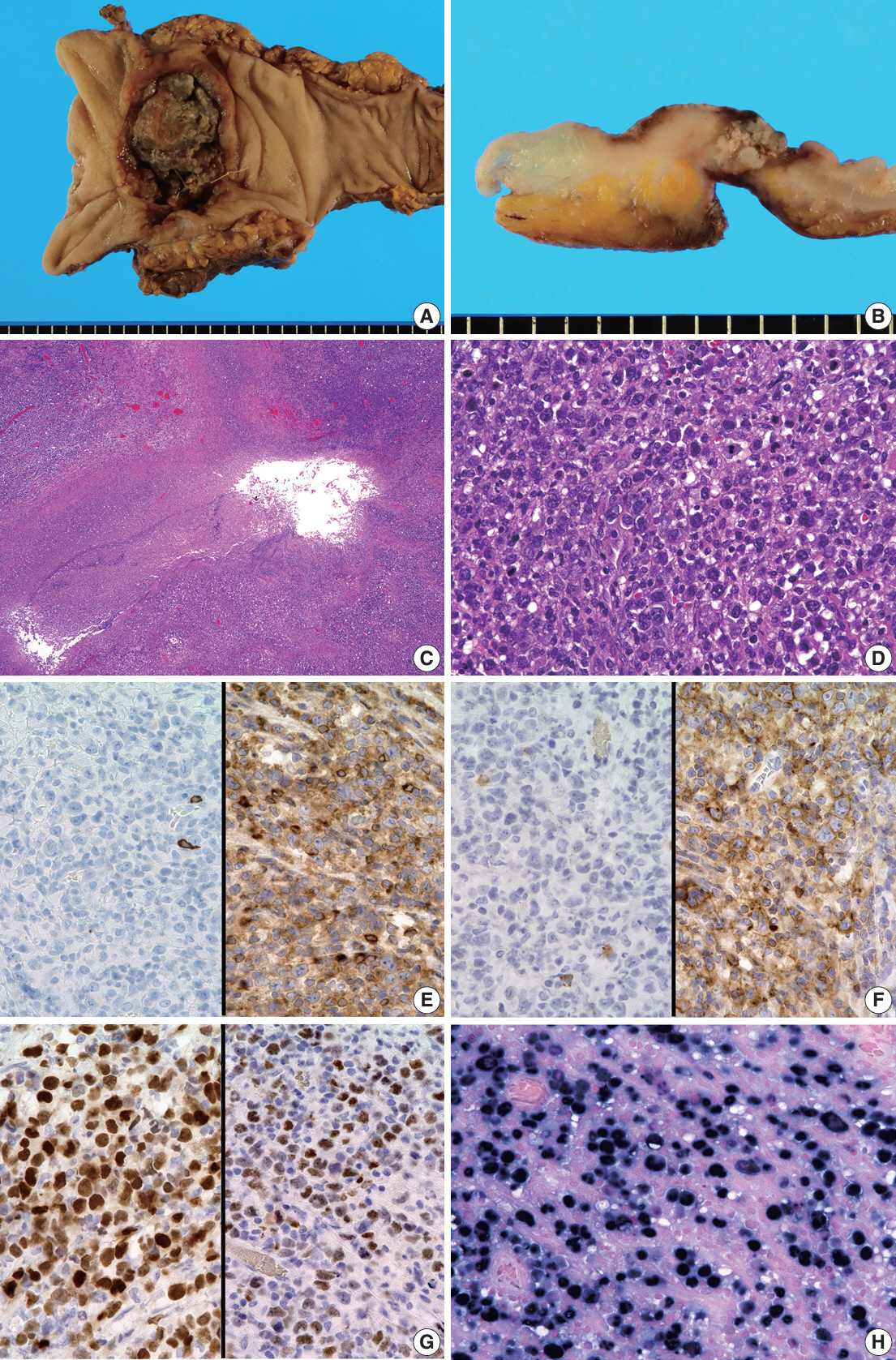
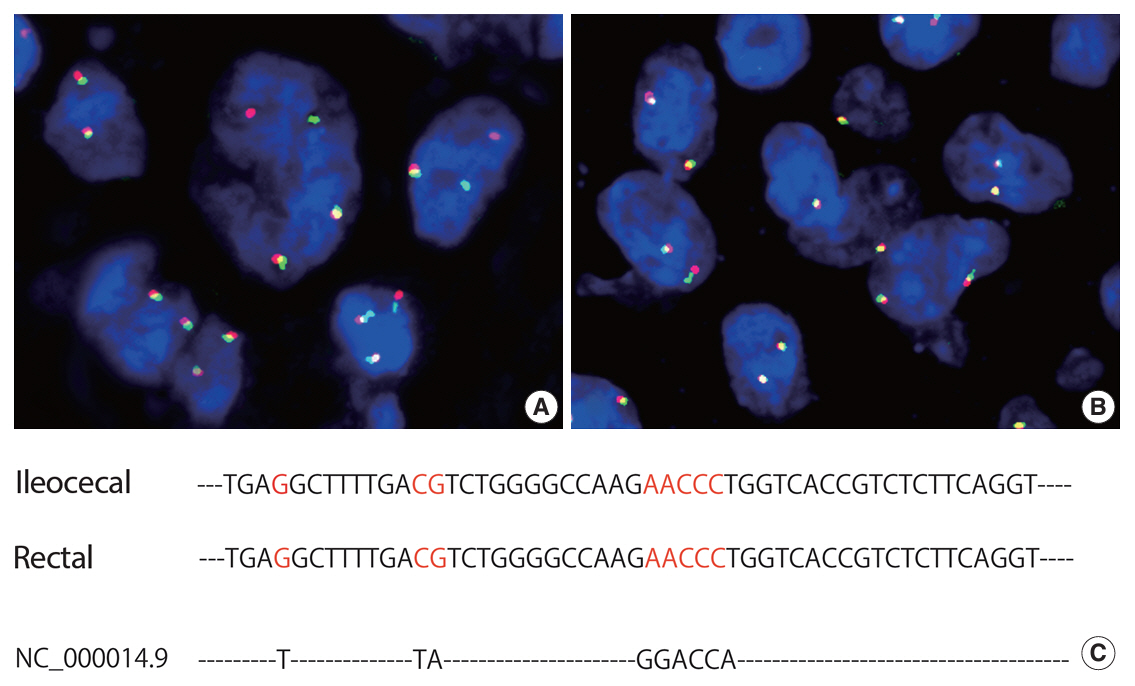
- Herein, we report a case of plasmablastic lymphoma (PBL) and diffuse large B-cell lymphoma (DLBCL) that occurred concurrently in the large intestine. An 84-year-old female presented with a palpable rectal tumor and ileocecal tumor observed on imaging analyses. Endoscopic biopsy of both lesions revealed lymphomatous round cells. Hartmann’s operation and ileocecal resection were performed for regional control. The ileocecal lesion consisted of a proliferation of CD20/CD79a-positive lymphoid cells, indicative of DLBCL. In contrast, the rectal tumor showed proliferation of atypical cells with pleomorphic nuclei and abundant amphophilic cytoplasm, with immunohistochemical findings of CD38/CD79a/MUM1/MYC (+) and CD20/CD3/CD138/PAX5 (–). Tumor cells were positive for Epstein-Barr virus– encoded RNA based on in situ hybridization and MYC rearrangement in fluorescence in situ hybridization analysis. These findings indicated the rectal tumor was most likely a PBL. Sequencing analysis for immunoglobulin heavy variable genes indicated a common B-cell origin of the two sets of lymphoma cells. This case report and literature review provide new insights into PBL tumorigenesis.
Keyword
Figure
Reference
-
References
1. Sarode SC, Sarode GS, Patil A. Plasmablastic lymphoma of the oral cavity: a review. Oral Oncol. 2010; 46:146–53.2. Campo E, Stein H, Harris NL. Plasmablastic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO classification of tumours of haematolymphoid and lymphoid tissues. Lyon: IARC Press;2017. p. 321–2.3. Kuppers R. The genomic landscape of HIV-associated plasmablastic lymphoma. Blood Cancer Discov. 2020; 1:23–5.4. Garcia-Reyero J, Martinez Magunacelaya N, Gonzalez de Villambrosia S, et al. Genetic lesions in MYC and STAT3 drive oncogenic transcription factor overexpression in plasmablastic lymphoma. Haematologica. 2021; 106:1120–8.5. Pather S, Mashele T, Willem P, et al. MYC status in HIV-associated plasmablastic lymphoma: dual-colour CISH, FISH and immunohistochemistry. Histopathology. 2021; 79:86–95.6. Marvyin K, Tjonnfjord EB, Breland UM, Tjonnfjord GE. Transformation to plasmablastic lymphoma in CLL upon ibrutinib treatment. BMJ Case Rep. 2020; 13:e235816.7. Ise M, Kageyama H, Ikebe D, Araki A, Kumagai K, Itami M. Transformation of double-hit follicular lymphoma to plasmablastic lymphoma: a partial role of MYC gene rearrangement. J Clin Exp Hematop. 2018; 58:128–35.8. Wu JZ, Min K, Fan L, et al. Plasmablastic lymphoma following combination treatment with fludarabine and rituximab for nongastric mucosa-associated lymphoid tissue lymphoma: a case report and review of literature. Int J Clin Exp Pathol. 2014; 7:4400–7.9. Hashimoto N, Ueda T, Hiraiwa S, Tajiri T, Nakamura N, Yokoyama K. Clonally related plasmablastic lymphoma simultaneously occurring with diffuse large B-cell lymphoma. Case Rep Hematol. 2020; 2020:8876567.10. Hatzimichael E, Papathanasiou K, Zerdes I, Flindris S, Papoudou-Bai A, Kapsali E. Plasmablastic lymphoma with coexistence of chronic lymphocytic leukemia in an immunocompetent patient: a case report and mini-review. Case Rep Hematol. 2017; 2017:2861596.11. Ronchi A, Marra L, Frigeri F, Botti G, Franco R, De Chiara A. Richter syndrome with plasmablastic lymphoma at primary diagnosis: a case report with a review of the literature. Appl Immunohistochem Mol Morphol. 2017; 25:e40–5.12. Holderness BM, Malhotra S, Levy NB, Danilov AV. Brentuximab vedotin demonstrates activity in a patient with plasmablastic lymphoma arising from a background of chronic lymphocytic leukemia. J Clin Oncol. 2013; 31:e197–9.13. Robak T, Urbanska-Rys H, Strzelecka B, et al. Plasmablastic lymphoma in a patient with chronic lymphocytic leukemia heavily pretreated with cladribine (2-CdA): an unusual variant of Richter’s syndrome. Eur J Haematol. 2001; 67:322–7.14. Foo WC, Huang Q, Sebastian S, Hutchinson CB, Burchette J, Wang E. Concurrent classical Hodgkin lymphoma and plasmablastic lymphoma in a patient with chronic lymphocytic leukemia/small lymphocytic lymphoma treated with fludarabine: a dimorphic presentation of iatrogenic immunodeficiency-associated lymphoproliferative disorder with evidence suggestive of multiclonal transformability of B cells by Epstein-Barr virus. Hum Pathol. 2010; 41:1802–8.15. Castillo JJ, Winer ES, Stachurski D, et al. Prognostic factors in chemotherapy-treated patients with HIV-associated plasmablastic lymphoma. Oncologist. 2010; 15:293–9.16. Witte HM, Kunstner A, Hertel N, et al. Integrative genomic and transcriptomic analysis in plasmablastic lymphoma identifies disruption of key regulatory pathways. Blood Adv. 2022; 6:637–51.17. Zelenetz AD, Gordon LI, Abramson JS, et al. NCCN Guidelines(R) Insights: B-cell lymphomas, version 6.2023. J Natl Compr Canc Netw. 2023; 21:1118–31.18. Bailly J, Jenkins N, Chetty D, Mohamed Z, Verburgh ER, Opie JJ. Plasmablastic lymphoma: an update. Int J Lab Hematol. 2022; 44 Suppl 1:54–63.19. Guerrero-Garcia TA, Mogollon RJ, Castillo JJ. Bortezomib in plasmablastic lymphoma: a glimpse of hope for a hard-to-treat disease. Leuk Res. 2017; 62:12–6.20. Castillo JJ, Guerrero-Garcia T, Baldini F, et al. Bortezomib plus EPOCH is effective as frontline treatment in patients with plasmablastic lymphoma. Br J Haematol. 2019; 184:679–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasmablastic Lymphoma in a Human Immunodeficiency Virus-negative Patient: A Case Report and Review of the Literature
- Relapse of Ocular Lymphoma following Primary Testicular Diffuse Large B-cell Lymphoma
- Primary Cutaneous T-cell/histiocyte-rich B-cell Lymphoma
- Diffuse Large B-cell Lymphoma in a Patient with Angioimmunoblastic T-cell Lymphoma
- Relationships among Hepatitis C Virus, Hepatocellular Carcinoma, and Diffuse Large B Cell Lymphoma: A Case Report