Cancer Res Treat.
2024 Jul;56(3):838-846. 10.4143/crt.2023.886.
Combined High-Dose Radiotherapy with Sequential Gemcitabine-Cisplatin Based Chemotherapy Increase the Resectability and Survival in Locally Advanced Unresectable Intrahepatic Cholangiocarcinoma: A Multi-institutional Cohort Study
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 2Depratment of Radiation Oncology, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- 3Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Center for Proton Therapy, National Cancer Center, Goyang, Korea
- 5Department of Radiation Oncology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 6Department of Radiation Oncology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2557670
- DOI: http://doi.org/10.4143/crt.2023.886
Abstract
- Purpose
The locally advanced unresectable intrahepatic cholangiocarcinoma (ICC) has detrimental oncological outcomes. In this study, we aimed to investigate the efficacy of radiotherapy in patients with locally advanced unresectable ICC.
Materials and Methods
Between 2001 and 2021, 116 patients were identified through medical record who underwent radiotherapy for locally advanced unresectable ICC. The resectability of ICC is determined by the multidisciplinary team at each institution. Overall survival (OS) were analyzed using the Kaplan-Meier method, and prognostic factors were analyzed using the Cox proportional hazards model.
Results
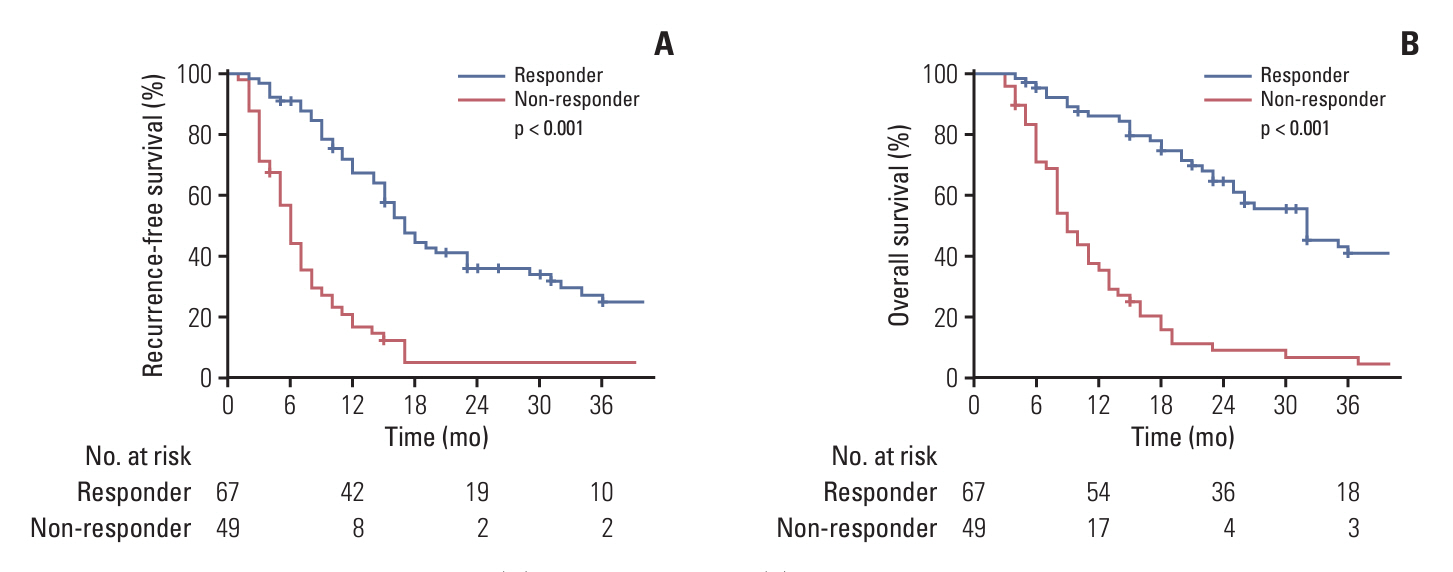
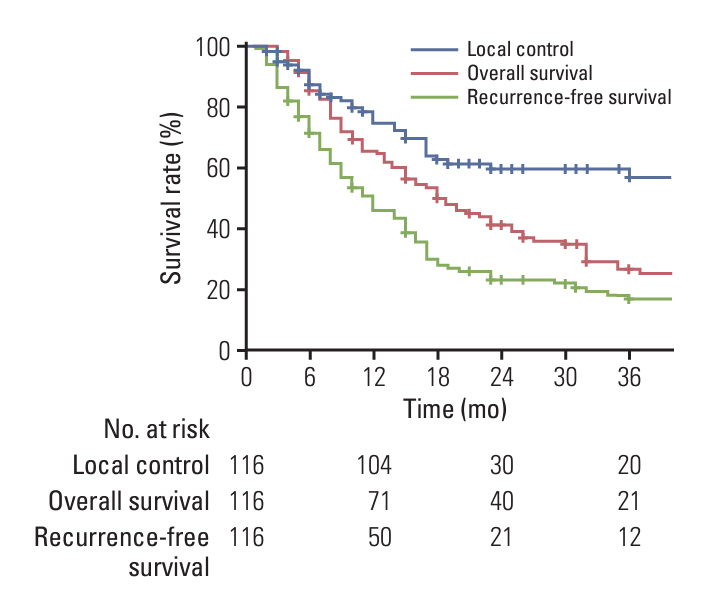
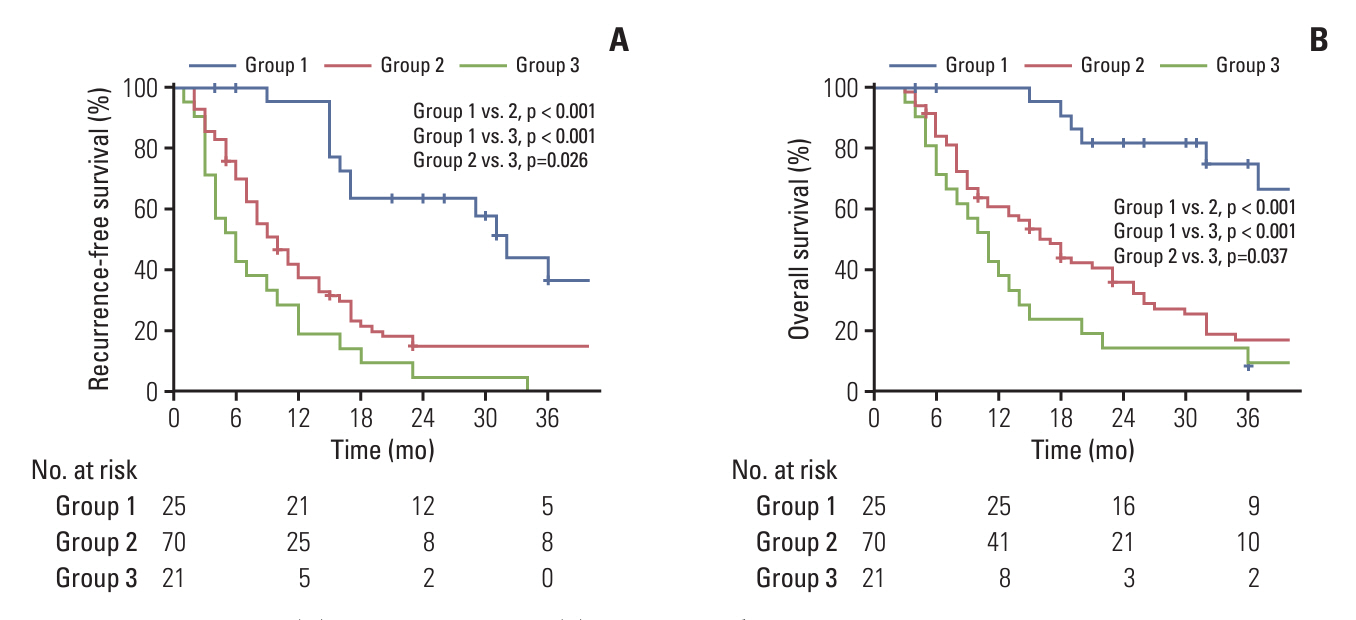
The median equivalent radiotherapy dose in 2 Gy fractions (EQD2) was 52 Gy (range, 30 to 110 Gy). Forty-seven patients (40.5%) received sequential gemcitabine-cisplatin based chemotherapy (GEM-CIS CTx). Multivariate analysis identified two risk factors, EQD2 of ≥ 60 Gy and application of sequential GEM-CIS CTx for OS. Patients were grouped by these two risk factors: group 1, EQD2 ≥ 60 Gy with sequential GEM-CIS CTx (n=25); group 2, EQD2 < 60 Gy with sequential GEM-CIS CTx or fluoropyrimidine-based concurrent chemoradiotherapy (n=70); and group 3, radiotherapy alone (n=21). Curative resection was more frequently undergone in group 1 than in groups 2 or 3 (28% vs. 8.6% vs. 0%, respectively). Consequently, OS was significantly better in group 1 than in groups 2 and 3 (p < 0.05).
Conclusion
Combined high-dose radiotherapy with sequential GEM-CIS CTx improved oncologic outcomes in patients with locally advanced unresectable ICC. Further prospective studies are required to validate these findings.
Figure
Reference
-
References
1. Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996; 7:593–600.
Article2. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–81.3. Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010; 103:469–74.
Article4. Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014; 25:391–8.
Article5. Oh DY, He AR, Qin S, Chen LT, Okusaka T, Vogel A, et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J Clin Oncol. 2022; 40(4 Suppl):378.
Article6. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023; 401:1853–65.7. Lamarca A, Ross P, Wasan HS, Hubner RA, McNamara MG, Lopes A, et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst. 2020; 112:200–10.
Article8. Yamashita S, Koay EJ, Passot G, Shroff R, Raghav KP, Conrad C, et al. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: a comprehensive analysis of 362 consecutive patients. Cancer. 2017; 123:1354–62.
Article9. Tao R, Krishnan S, Bhosale PR, Javle MM, Aloia TA, Shroff RT, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol. 2016; 34:219–26.
Article10. Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016; 34:460–8.
Article11. De B, Tran Cao HS, Vauthey JN, Manzar GS, Corrigan KL, Raghav KP, et al. Ablative liver radiotherapy for unresected intrahepatic cholangiocarcinoma: patterns of care and survival in the United States. Cancer. 2022; 128:2529–39.
Article12. Smart AC, Goyal L, Horick N, Petkovska N, Zhu AX, Ferrone CR, et al. Hypofractionated radiation therapy for unresectable/locally recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2020; 27:1122–9.13. Apisarnthanarax S, Barry A, Cao M, Czito B, DeMatteo R, Drinane M, et al. External beam radiation therapy for primary liver cancers: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2022; 12:28–51.
Article14. Chang WW, Hsiao PK, Qin L, Chang CL, Chow JM, Wu SY. Treatment outcomes for unresectable intrahepatic cholangiocarcinoma: nationwide, population-based, cohort study based on propensity score matching with the Mahalanobis metric. Radiother Oncol. 2018; 129:284–92.
Article15. Jackson MW, Amini A, Jones BL, Rusthoven CG, Schefter TE, Goodman KA. Treatment selection and survival outcomes with and without radiation for unresectable, localized intrahepatic cholangiocarcinoma. Cancer J. 2016; 22:237–42.
Article16. Kim YI, Park JW, Kim BH, Woo SM, Kim TH, Koh YH, et al. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for advanced-stage unresectable intrahepatic cholangiocarcinoma. Radiat Oncol. 2013; 8:292.
Article17. Verma V, Kusi Appiah A, Lautenschlaeger T, Adeberg S, Simone CB 2nd, Lin C. Chemoradiotherapy versus chemotherapy alone for unresected intrahepatic cholangiocarcinoma: practice patterns and outcomes from the national cancer data base. J Gastrointest Oncol. 2018; 9:527–35.
Article18. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014; 60:1268–89.
Article19. Guro H, Kim JW, Choi Y, Cho JY, Yoon YS, Han HS. Multidisciplinary management of intrahepatic cholangiocarcinoma: Current approaches. Surg Oncol. 2017; 26:146–52.
Article20. Joiner MC. A simple alpha/beta-independent method to derive fully isoeffective schedules following changes in dose per fraction. Int J Radiat Oncol Biol Phys. 2004; 58:871–5.21. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.
Article22. Sebastian NT, Tan Y, Miller ED, Williams TM, Noonan AM, Hays JL, et al. Association of liver-directed local therapy with overall survival in adults with metastatic intrahepatic cholangiocarcinoma. JAMA Netw Open. 2019; 2:e1911154.23. Brunner TB, Blanck O, Lewitzki V, Abbasi-Senger N, Momm F, Riesterer O, et al. Stereotactic body radiotherapy dose and its impact on local control and overall survival of patients for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. Radiother Oncol. 2019; 132:42–7.
Article24. Kozak MM, Toesca DAS, von Eyben R, Pollom EL, Chang DT. Stereotactic body radiation therapy for cholangiocarcinoma: optimizing locoregional control with elective nodal irradiation. Adv Radiat Oncol. 2020; 5:77–84.
Article25. Ohkawa A, Mizumoto M, Ishikawa H, Abei M, Fukuda K, Hashimoto T, et al. Proton beam therapy for unresectable intrahepatic cholangiocarcinoma. J Gastroenterol Hepatol. 2015; 30:957–63.
Article26. Shroff RT, Guthrie KA, Scott AJ, Borad MJ, Goff LW, Matin K, et al. SWOG 1815: a phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol. 2023; 41(4 Suppl):LBA490.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathologic Complete Remission in a Patient with Locally Advanced Unresectable Intrahepatic Cholangiocarcinoma Treated with Chemotherapy
- Long Term Complete Response of Unresectable Locally Advanced Pancreatic Cancer after CCRT and Gemcitabine Chemotherapy
- The Results of Radiotherapy in Locally Advanced, Unresectable Pancreatic Cancer
- Novel Palliative Chemotherapy for Cholangiocarcinoma
- Gemcitabine-based Chemotherapy for Gallbladder Cancer