Cancer Res Treat.
2024 Jul;56(3):751-764. 10.4143/crt.2023.1118.
Association of Immune-Related Adverse Events and the Efficacy of Anti–PD-(L)1 Monotherapy in Non–Small Cell Lung Cancer: Adjusting for Immortal-Time Bias
- Affiliations
-
- 1Department of Thoracic Medical Oncology, Zhejiang Cancer Hospital, Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences, Hangzhou, China
- 2Department of Oncology, The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 3Wenzhou Medical University, Wenzhou, China
- KMID: 2557662
- DOI: http://doi.org/10.4143/crt.2023.1118
Abstract
- Purpose
The association between immune-related adverse events (irAEs) and survival outcomes in non–small cell lung cancer (NSCLC) patients treated with programmed death-(ligand) 1 [PD-(L)1] inhibitors remains controversial, partly due to variations in dealing with immortal-time bias (ITB).
Materials and Methods
We retrospectively enrolled 425 advanced NSCLC patients who received anti–PD-(L)1 monotherapy between January 2016 and June 2021, stratifying them into irAE (n=127) and non-irAE (n=298) groups. The primary endpoint was to assess the impact of irAEs on progression-free survival (PFS) and overall survival (OS). Landmark (2-, 3-, 6-, and 9-month) and time-dependent Cox analyses were performed to eliminate ITB.
Results
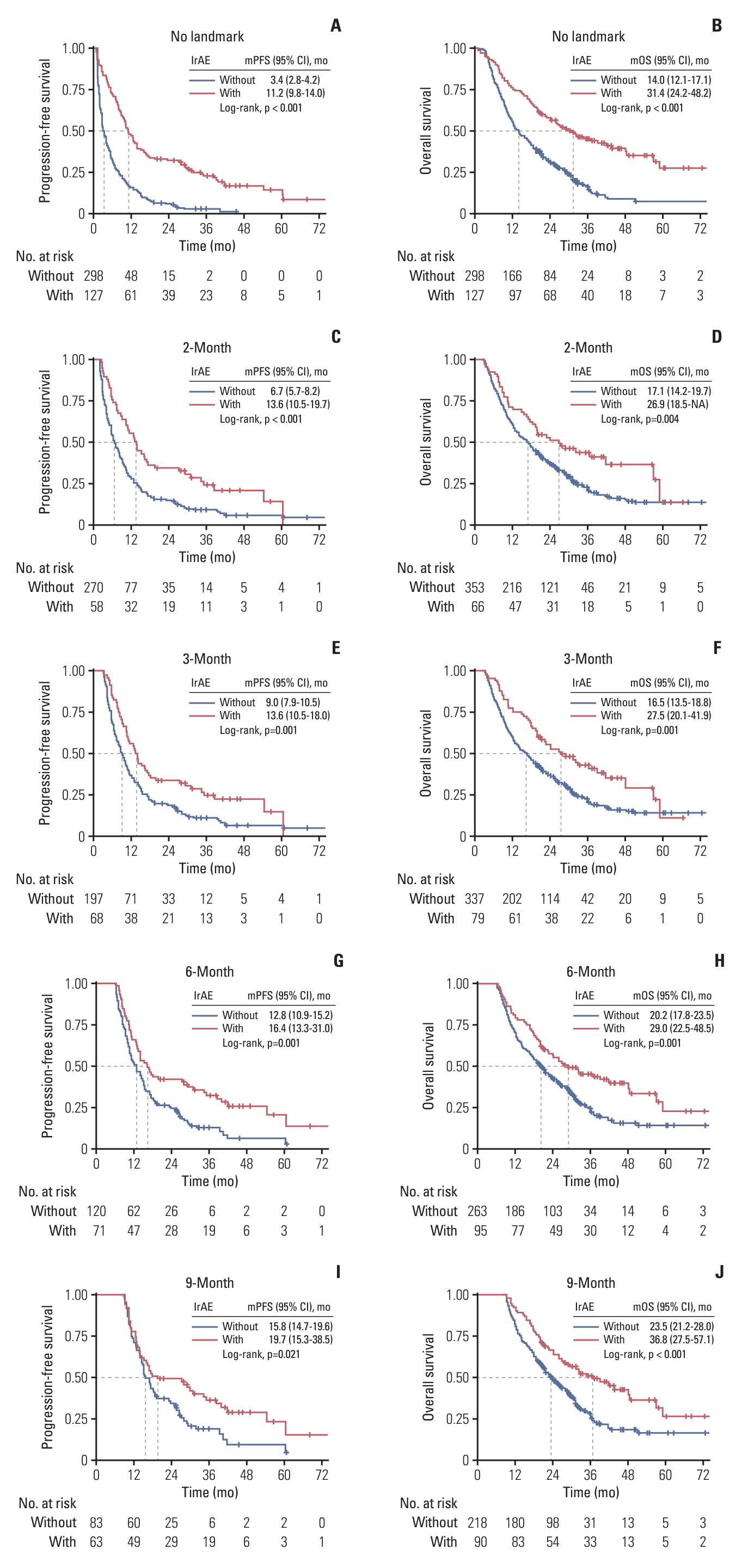
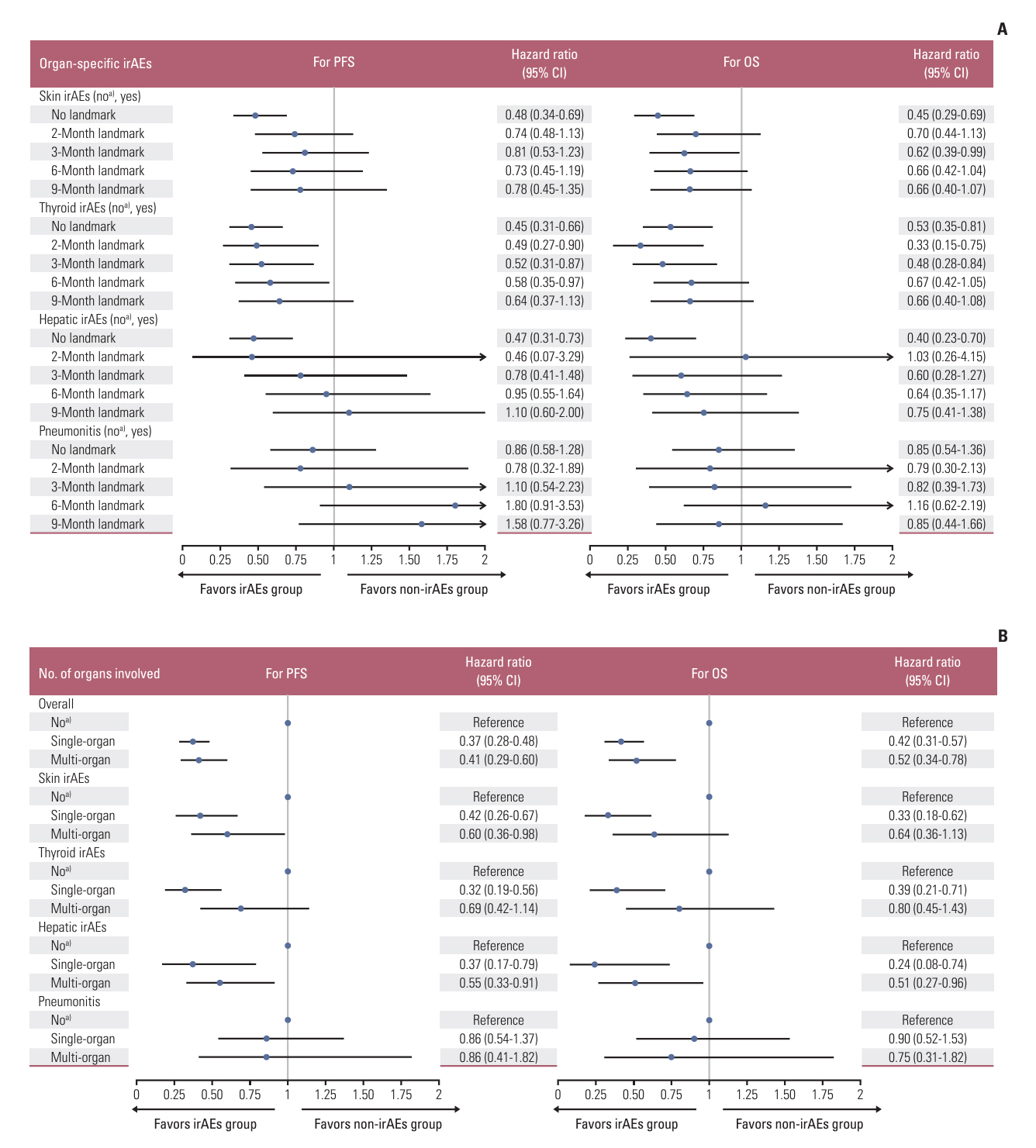
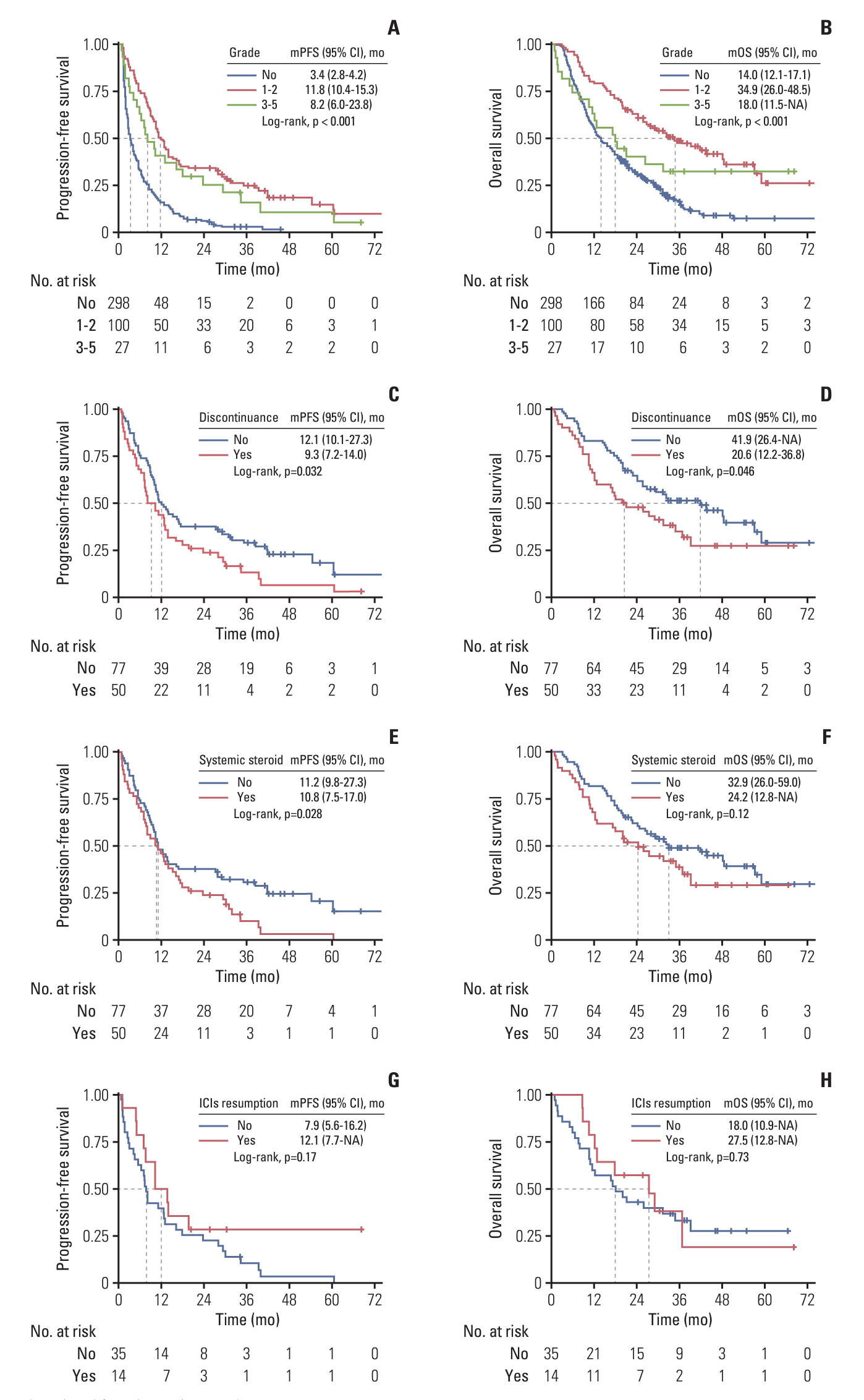
With a median follow-up of 38.8 months, the occurrence of overall irAEs was significantly associated with superior PFS (11.2 vs. 3.4 months, p < 0.001) and OS (31.4 vs. 14.0 months, p < 0.001), which persisted in landmark and time-dependent Cox analyses. For the main organ-specific irAEs, skin, thyroid, and hepatic irAEs, respectively, showed significantly improved survival compared to the non-irAE group, whereas pneumonitis did not. Single-organ irAEs had the best outcomes compared with multi-organ or no irAE, which also held across subgroups of skin, thyroid, and hepatic irAEs. Moreover, severe grade irAEs and immunotherapy discontinuation had a detrimental effect on survival, systemic steroid therapy showed little effect, while immunotherapy resumption had tolerable safety and a trend of improved survival.
Conclusion
After adequately adjusting ITB, the occurrence of overall irAEs predicts for favorable efficacy of anti–PD-(L)1 monotherapy in NSCLC, with better outcomes observed in patients with skin, thyroid, or hepatic irAEs, particularly those with single-organ involvement.
Keyword
Figure
Reference
-
References
1. Berner F, Bomze D, Diem S, Ali OH, Fassler M, Ring S, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 2019; 5:1043–7.
Article2. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling preexisting antibodies in patients treated with anti-PD-1 therapy for advanced non-small cell lung cancer. JAMA Oncol. 2019; 5:376–83.
Article3. de Moel EC, Rozeman EA, Kapiteijn EH, Verdegaal EM, Grummels A, Bakker JA, et al. Autoantibody development under treatment with immune-checkpoint inhibitors. Cancer Immunol Res. 2019; 7:6–11.
Article4. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018; 378:158–68.5. Kang JH, Bluestone JA, Young A. Predicting and preventing immune checkpoint inhibitor toxicity: targeting cytokines. Trends Immunol. 2021; 42:293–311.
Article6. Rogado J, Sanchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Levi A, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer. 2019; 109:21–7.
Article7. Schweizer C, Schubert P, Rutzner S, Eckstein M, Haderlein M, Lettmaier S, et al. Prospective evaluation of the prognostic value of immune-related adverse events in patients with nonmelanoma solid tumour treated with PD-1/PD-L1 inhibitors alone and in combination with radiotherapy. Eur J Cancer. 2020; 140:55–62.
Article8. Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol. 2017; 12:1798–805.9. Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013; 31:2963–9.
Article10. Maillet D, Corbaux P, Stelmes JJ, Dalle S, Locatelli-Sanchez M, Perier-Muzet M, et al. Association between immune-related adverse events and long-term survival outcomes in patients treated with immune checkpoint inhibitors. Eur J Cancer. 2020; 132:61–70.
Article11. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018; 4:374–8.12. Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018; 115:71–4.
Article13. Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Kim YH, Tomii K, et al. Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: a multicenter retrospective cohort study. Lung Cancer. 2018; 119:14–20.
Article14. Kfoury M, Najean M, Lappara A, Voisin AL, Champiat S, Michot JM, et al. Analysis of the association between prospectively collected immune-related adverse events and survival in patients with solid tumor treated with immune-checkpoint blockers, taking into account immortal-time bias. Cancer Treat Rev. 2022; 110:102452.
Article15. Socinski MA, Jotte RM, Cappuzzo F, Nishio M, Mok TS, Reck M, et al. Association of immune-related adverse events with efficacy of atezolizumab in patients with non-small cell lung cancer: pooled analyses of the phase 3 IMpower130, IMpower132, and IMpower150 randomized clinical trials. JAMA Oncol. 2023; 9:527–35.
Article16. Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017; 28:583–9.
Article17. Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020; 6:1952–6.18. Lisberg A, Tucker DA, Goldman JW, Wolf B, Carroll J, Hardy A, et al. Treatment-related adverse events predict improved clinical outcome in NSCLC patients on KEYNOTE-001 at a single center. Cancer Immunol Res. 2018; 6:288–94.
Article19. Wang W, Gu X, Wang L, Pu X, Feng H, Xu C, et al. The prognostic impact of mild and severe immune-related adverse events in non-small cell lung cancer treated with immune checkpoint inhibitors: a multicenter retrospective study. Cancer Immunol Immunother. 2022; 71:1693–703.
Article20. Kurokawa K, Mitsuishi Y, Shimada N, Kawakami Y, Miura K, Miyawaki T, et al. Association between the efficacy and immune-related adverse events of pembrolizumab and chemotherapy in non-small cell lung cancer patients: a retrospective study. BMC Cancer. 2022; 22:1047.
Article21. Cheung YM, Wang W, McGregor B, Hamnvik OR. Associations between immune-related thyroid dysfunction and efficacy of immune checkpoint inhibitors: a systematic review and meta-analysis. Cancer Immunol Immunother. 2022; 71:1795–812.
Article22. Beaufils M, Amodru V, Tejeda M, Boher JM, Zemmour C, Chanez B, et al. Dysthyroidism during immune checkpoint inhibitors is associated with improved overall survival in adult cancers: data mining of 1385 electronic patient records. J Immunother Cancer. 2023; 11:e006786.23. Suresh K, Psoter KJ, Voong KR, Shankar B, Forde PM, Ettinger DS, et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thorac Oncol. 2019; 14:494–502.
Article24. Naqash AR, Ricciuti B, Owen DH, Florou V, Toi Y, Cherry C, et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother. 2020; 69:1177–87.
Article25. Verheijden RJ, van Eijs MJ, May AM, van Wijk F, Suijkerbuijk KP. Immunosuppression for immune-related adverse events during checkpoint inhibition: an intricate balance. NPJ Precis Oncol. 2023; 7:41.
Article26. Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020; 17:504–15.
Article27. Henderson Berg MH, Del Rincon SV, Miller WH. Potential therapies for immune-related adverse events associated with immune checkpoint inhibition: from monoclonal antibodies to kinase inhibition. J Immunother Cancer. 2022; 10:e003551.28. Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 2019; 5:1310–7.
Article29. Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018; 6:1093–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peripheral blood immune cell-based biomarkers in anti-PD-1/PD-L1 therapy
- Cutaneous Adverse Events of Anti-Programmed Cell Death Receptor-1 Antibody: Two Case Reports and a Literature Review
- A Case of Aggravation of Thyroid Goiter after Treatment with PD-1 Inhibitor for Breast Cancer in Patients with Underlying Hashimoto's Thyroiditis
- Characteristics of Immune-Related Thyroid Adverse Events in Patients Treated with PD-1/PD-L1 Inhibitors
- Immune Checkpoint Inhibitors in 10 Years: Contribution of Basic Research and Clinical Application in Cancer Immunotherapy