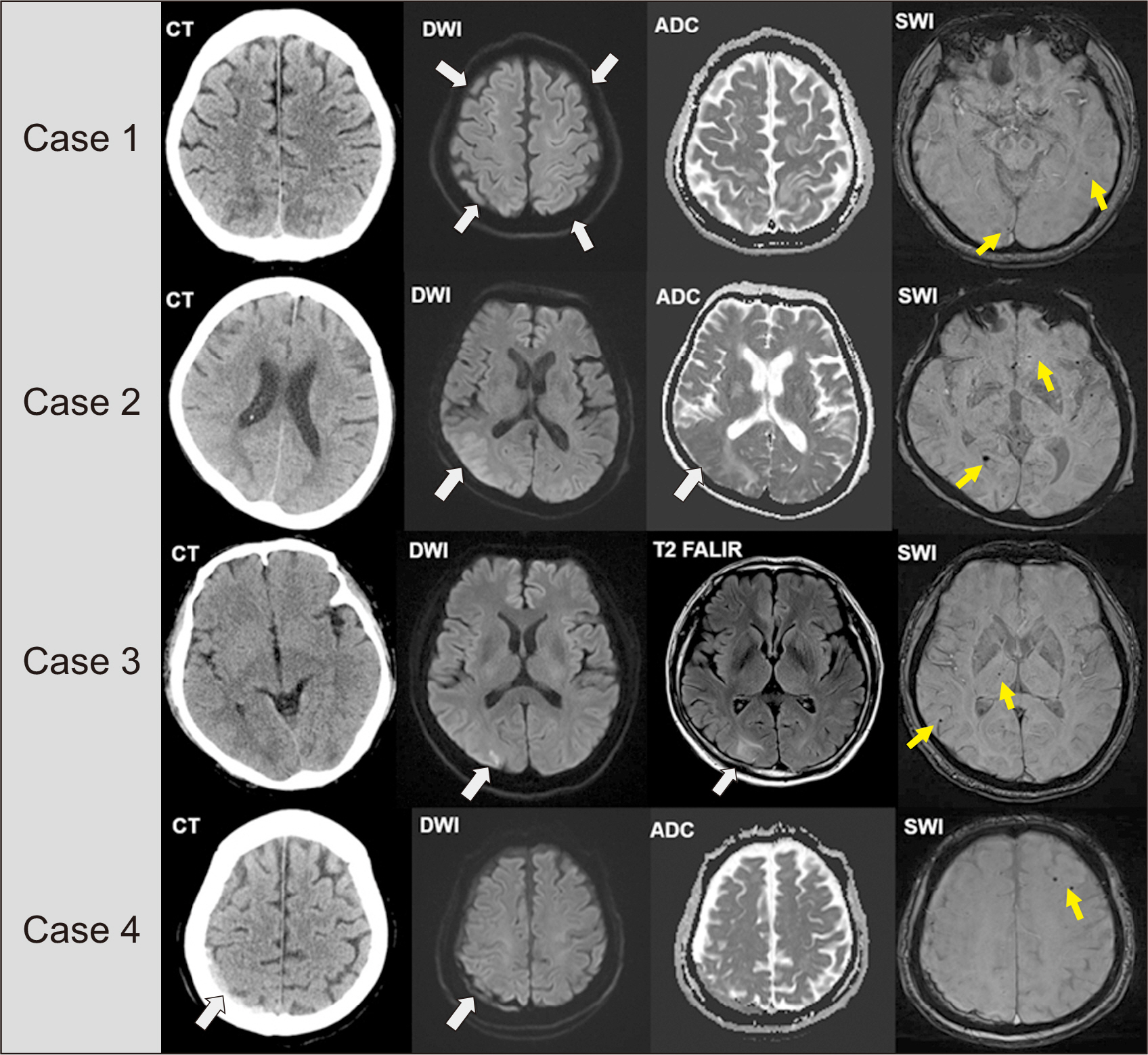
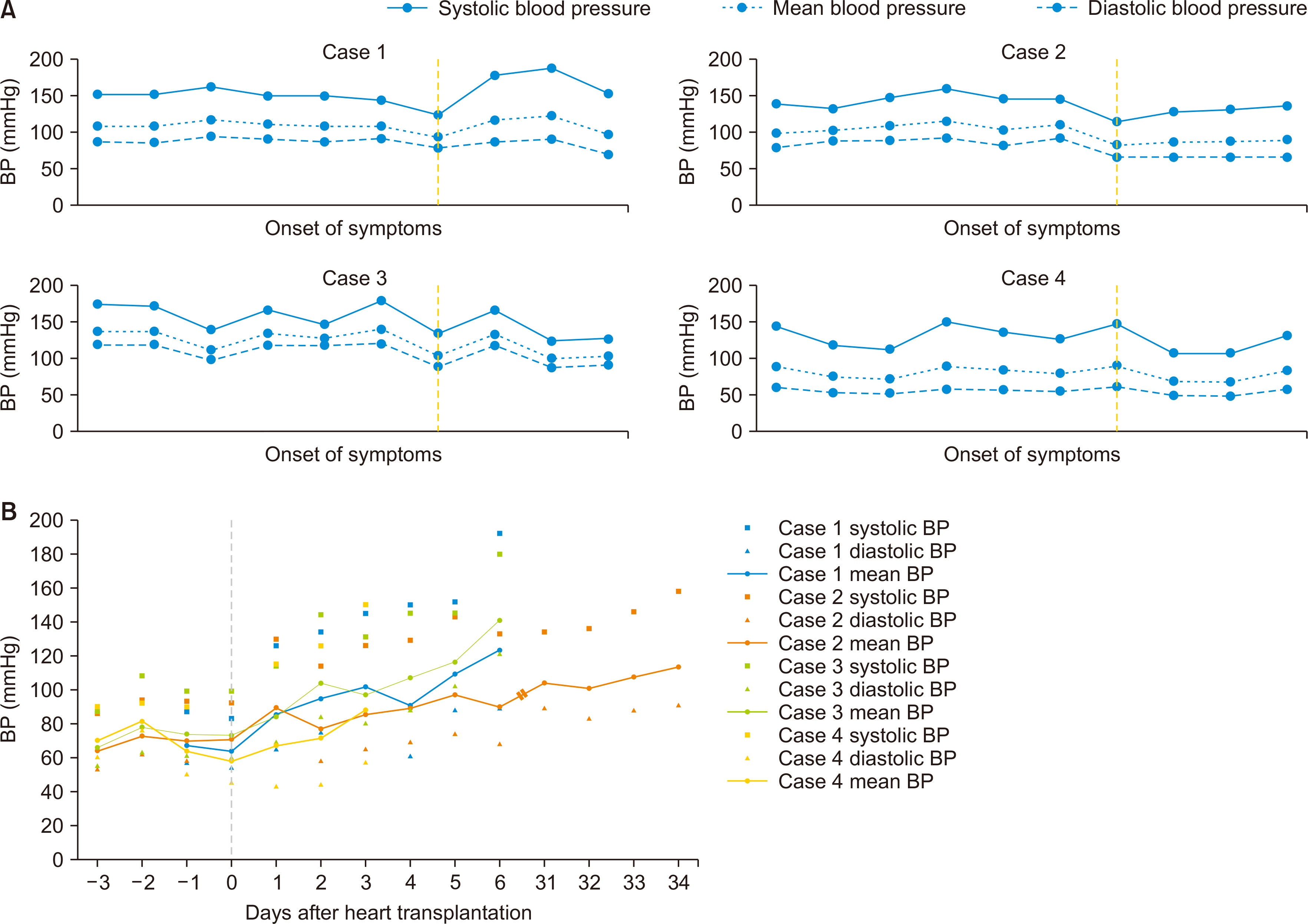
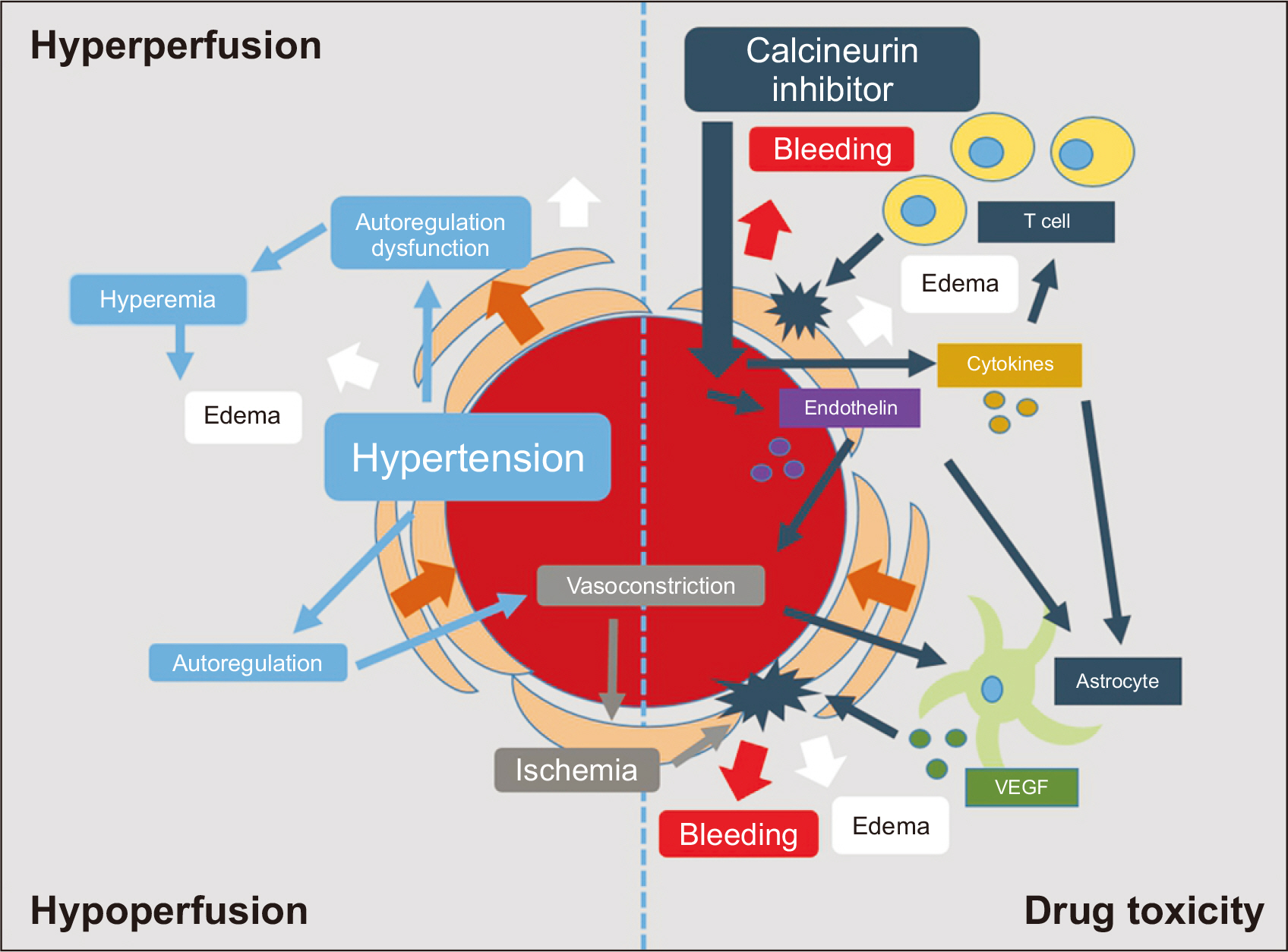
Clin Transplant Res.
2024 Jun;38(2):154-162. 10.4285/ctr.24.0009.
Clinical features and outcomes of posterior reversible encephalopathy syndrome after heart transplantation: a case series
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine and Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- 2Department of Neurology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- KMID: 2557610
- DOI: http://doi.org/10.4285/ctr.24.0009
Abstract
- Posterior reversible encephalopathy syndrome (PRES) is a rare neurological disease that may be associated with hypertension, autoregulatory failure, and the use of calcineurin inhibitors following heart transplantation (HT). In this article, we present a case series of PRES, discussing its potential causes and management strategies. Among the 126 HT recipients at our hospital, four were diagnosed with PRES. Three of these patients developed PRES within 7 days after HT. Prior to the onset of PRES, all patients experienced sustained hypertension, and strict blood pressure (BP) control was maintained. Three of the four patients recovered without PRES recurrence, while one patient died of sepsis after an episode of altered consciousness. Hypertension was observed in all patients prior to the onset of PRES, and the majority experienced symptom improvement with BP control. While most cases of PRES were reversible with conservative treatment, including the administration of antiepileptics, one irreversible case resulted in in-hospital mortality. Thus, PRES can have serious outcomes and is not invariably benign.
Keyword
Figure
Reference
-
1. Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, et al. 2017; The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 36:1037–46. DOI: 10.1016/j.healun.2017.07.019. PMID: 28779893.
Article2. van de Beek D, Kremers W, Daly RC, Edwards BS, Clavell AL, McGregor CG, et al. 2008; Effect of neurologic complications on outcome after heart transplant. Arch Neurol. 65:226–31. DOI: 10.1001/archneurol.2007.52. PMID: 18268192.
Article3. Pruitt AA. 2017; Neurologic complications of transplantation. Continuum (Minneap Minn). 23:802–21. DOI: 10.1212/CON.0000000000000473. PMID: 28570330.
Article4. Bartynski WS. 2008; Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 29:1043–9. DOI: 10.3174/ajnr.A0929. PMID: 18403560. PMCID: PMC8118813.
Article5. Casey SO, Sampaio RC, Michel E, Truwit CL. 2000; Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol. 21:1199–206. PMID: 10954269. PMCID: PMC8174901.6. Wu Q, Marescaux C, Wolff V, Jeung MY, Kessler R, Lauer V, et al. 2010; Tacrolimus-associated posterior reversible encephalopathy syndrome after solid organ transplantation. Eur Neurol. 64:169–77. DOI: 10.1159/000319032. PMID: 20699617.
Article7. Schwartz RB, Bravo SM, Klufas RA, Hsu L, Barnes PD, Robson CD, et al. 1995; Cyclosporine neurotoxicity and its relationship to hypertensive encephalopathy: CT and MR findings in 16 cases. AJR Am J Roentgenol. 165:627–31. DOI: 10.2214/ajr.165.3.7645483. PMID: 7645483.
Article8. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. 1996; A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 334:494–500. DOI: 10.1056/NEJM199602223340803. PMID: 8559202.
Article9. Fugate JE, Rabinstein AA. 2015; Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 14:914–25. DOI: 10.1016/S1474-4422(15)00111-8. PMID: 26184985.10. Bartynski WS, Tan HP, Boardman JF, Shapiro R, Marsh JW. 2008; Posterior reversible encephalopathy syndrome after solid organ transplantation. AJNR Am J Neuroradiol. 29:924–30. DOI: 10.3174/ajnr.A0960. PMID: 18272559. PMCID: PMC8128592.
Article11. Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. 2010; Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 85:427–32. DOI: 10.4065/mcp.2009.0590. PMID: 20435835. PMCID: PMC2861971.
Article12. Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. 2009; Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. Am J Neurorad. 30:1371–79. DOI: 10.3174/ajnr.A1588. PMID: 19386731. PMCID: PMC7051550.
Article13. Kapoor A, Birks E, Lenneman A, McCants K. 2017; Posterior reversible encephalopathy syndrome after heart transplantation: diagnosis and immunosuppressive therapy. Tex Heart Inst J. 44:205–8. DOI: 10.14503/THIJ-15-5007. PMID: 28761402. PMCID: PMC5505400.14. Schwartz RB, Jones KM, Kalina P, Bajakian RL, Mantello MT, Garada B, et al. 1992; Hypertensive encephalopathy: findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol. 159:379–83. DOI: 10.2214/ajr.159.2.1632361. PMID: 1632361.15. Textor SC, Taler SJ, Canzanello VJ, Schwartz L, Augustine JE. 2000; Posttransplantation hypertension related to calcineurin inhibitors. Liver Transpl. 6:521–30. DOI: 10.1053/jlts.2000.9737. PMID: 10980050.
Article16. Marsden PA, Brenner BM. 1992; Transcriptional regulation of the endothelin-1 gene by TNF-alpha. Am J Physiol. 262(4 Pt 1):C854–61. DOI: 10.1152/ajpcell.1992.262.4.C854. PMID: 1566813.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Posterior Reversible Encephalopathy Syndrome
- Posterior Reversible Encephalopathy Syndrome in a Patient with Intoxication of Arisaema amurense
- Malignant Posterior Reversible Encephalopathy Syndrome: A Case of Posterior Irreversible Encephalopathy Syndrome
- Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome associated with acute exacerbation of chronic obstructive pulmonary disease
- Reversible Cerebral Vasoconstriction Syndrome and Posterior Reversible Encephalopathy Syndrome Presenting with Deep Intracerebral Hemorrhage in Young Women