Acute Crit Care.
2024 May;39(2):251-256. 10.4266/acc.2023.01046.
Are sodium-glucose co-transporter-2 inhibitors associated with improved outcomes in diabetic patients admitted to intensive care units with septic shock?
- Affiliations
-
- 1Department of Internal Medicine, Mayo Clinic, Scottsdale, AZ, USA
- 2Department of Pulmonary and Critical Care, Mayo Clinic, Scottsdale, AZ, USA
- 3Department of Quantitative Health Science Research, Mayo Clinic, Scottsdale, AZ, USA
- KMID: 2557241
- DOI: http://doi.org/10.4266/acc.2023.01046
Abstract
- Background
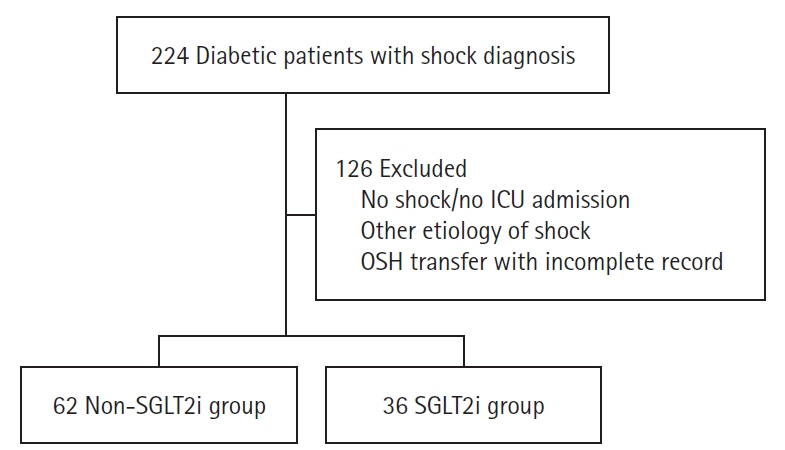
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) have been shown to reduce organ dysfunction in renal and cardiovascular disease. There are limited data on the role of SGLT2i in acute organ dysfunction. We conducted a study to assess the effect of SGLT2i taken prior to intensive care unit (ICU) admission in diabetic patients admitted with septic shock. Methods: This retrospective cohort study used electronic medical records and included diabetic patients admitted to the ICU with septic shock. We compared diabetic patients on SGLT2i to those who were not on SGLT2i prior to admission. The primary outcome was in-hospital mortality, and secondary outcomes included hospital and ICU length of stay, use of renal replacement therapy, and 28- and 90-day mortality. Results: A total of 98 diabetic patients was included in the study, 36 in the SGLT2i group and 62 in the non-SGLT2i group. The Sequential Organ Failure Assessment and Acute Physiology and Chronic Health Evaluation III scores were similar in the groups. Inpatient mortality was significantly lower in the SGLT2i group (5.6% vs. 27.4%, P=0.008). There was no significant difference in secondary outcomes. Conclusions: Our study found that diabetic patients on SGLT2i prior to hospitalization who were admitted to the ICU with septic shock had lower inpatient mortality compared to patients not on SGLT2i.
Keyword
Figure
Reference
-
1. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001; 29:1303–10.
Article2. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021; 47:1181–247.3. Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018; 378:797–808.
Article4. Kosiborod MN, Esterline R, Furtado RH, Oscarsson J, Gasparyan SB, Koch GG, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021; 9:586–94.
Article5. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383:1413–24.6. Heerspink HJ, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383:1436–46.
Article7. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385:1451–61.8. The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023; 388:117–27.
Article9. Angé M, De Poortere J, Ginion A, Battault S, Dechamps M, Muccioli GG, et al. Canagliflozin protects against sepsis capillary leak syndrome by activating endothelial α1AMPK. Sci Rep. 2021; 11:13700.
Article10. Lopaschuk GD, Verma S. cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020; 5:632–44.11. Mancini SJ, Boyd D, Katwan OJ, Strembitska A, Almabrouk TA, Kennedy S, et al. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep. 2018; 8:5276.
Article12. Huang J, Liu K, Zhu S, Xie M, Kang R, Cao L, et al. AMPK regulates immunometabolism in sepsis. Brain Behav Immun. 2018; 72:89–100.
Article13. Yau K, Dharia A, Alrowiyti I, Cherney DZ. Prescribing SGLT2 inhibitors in patients with CKD: expanding indications and practical considerations. Kidney Int Rep. 2022; 7:1463–76.
Article14. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022; 102(5S):S1–127.15. Silverii GA, Dicembrini I, Monami M, Mannucci E. Fournier’s gangrene and sodium-glucose co-transporter-2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2020; 22:272–5.
Article16. Beitelshees AL, Leslie BR, Taylor SI. Sodium-glucose cotransporter 2 inhibitors: a case study in translational research. Diabetes. 2019; 68:1109–20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SGLT2 Inhibitors and Ketoacidosis: Pathophysiology and Management
- Response: Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors (Diabetes Metab J 2019;43:158–73)
- Letter: Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors (Diabetes Metab J 2019;43:158–73)
- Advances in the management of diabetic kidney disease: beyond sodium-glucose co-transporter 2 inhibitors
- Sodium-Glucose Cotransporter 2 Inhibitors for Chronic Kidney Disease: A Comprehensive Review