Korean J healthc assoc Infect Control Prev.
2024 Jun;29(1):59-62. 10.14192/kjicp.2024.29.1.59.
Impact of the Rapid COVID-19 Polymerase Chain Reaction Test on Length of Emergency Room Stay
- Affiliations
-
- 1Department of Laboratory Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- KMID: 2556881
- DOI: http://doi.org/10.14192/kjicp.2024.29.1.59
Abstract
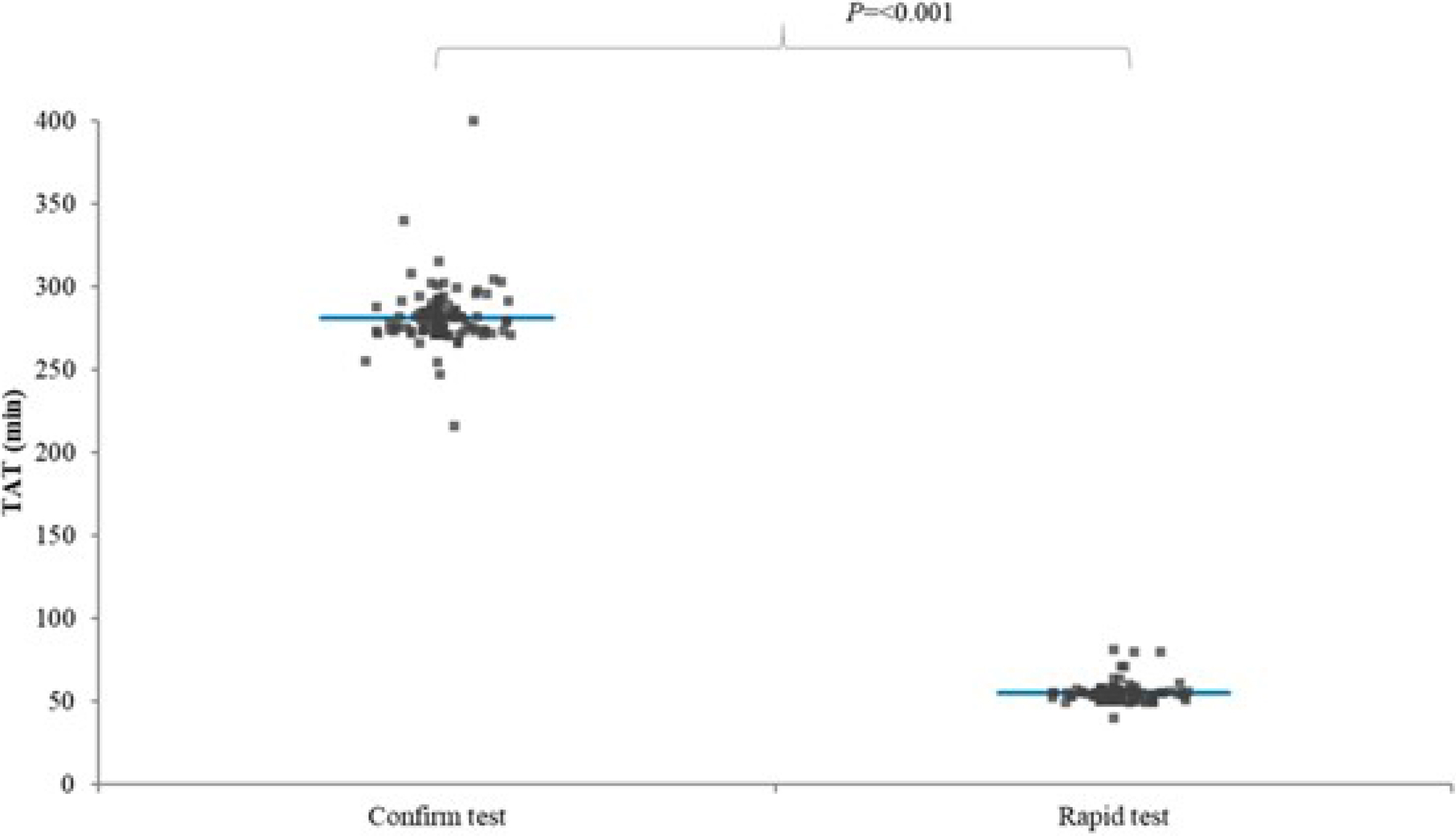
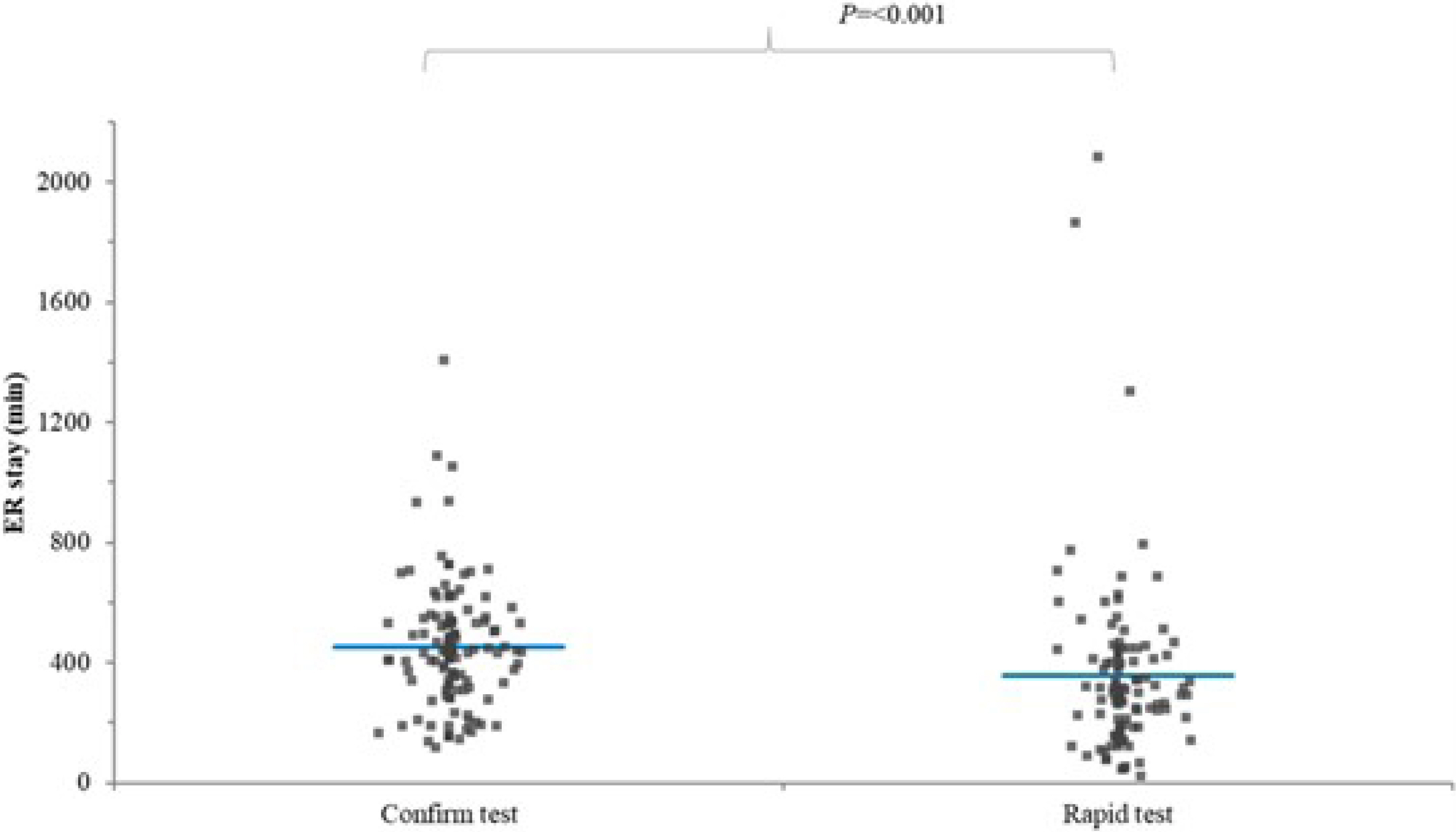
- The rapid COVID-19 polymerase chain reaction (PCR) test using Xpert Xpress SARS-CoV-2 and the conventional COVID-19 PCR test using Standard M nCoV Detection were compared after matching for sex, age, and hospitalization period. The rapid COVID-19 PCR test group exhibited shorter turnaround time and emergency department stay.
Figure
Reference
-
1. Ochani R, Asad A, Yasmin F, Shaikh S, Khalid H, Batra S, et al. 2021; COVID-19 pandemic: from origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med. 29:20–36.2. Tang YW, Schmitz JE, Persing DH, Stratton CW. 2020; Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol. 58:e00512–20. DOI: 10.1128/JCM.00512-20. PMID: 32245835. PMCID: PMC7269383.3. Rödel J, Egerer R, Suleyman A, Sommer-Schmid B, Baier M, Henke A, et al. 2020; Use of the variplexTM SARS-CoV-2 RT-LAMP as a rapid molecular assay to complement RT-PCR for COVID-19 diagnosis. J Clin Virol. 132:104616. DOI: 10.1016/j.jcv.2020.104616. PMID: 32891938. PMCID: PMC7457909.4. Bruijns B, Folkertsma L, Tiggelaar R. 2022; FDA authorized molecular point-of-care SARS-CoV-2 tests: a critical review on principles, systems and clinical performances. Biosens Bioelectron X. 11:100158. DOI: 10.1016/j.biosx.2022.100158. PMID: 35619623. PMCID: PMC9122839.5. Kim M, Kim HS, Bae HG, Huh HJ, Sung H. 2021; Laboratory diagnosis and utilization for COVID-19. Korean J healthc assoc Infect Control Prev. 26:47–56. DOI: 10.14192/kjicp.2021.26.2.47.6. Hur KH, Park K, Lim Y, Jeong YS, Sung H, Kim MN. 2020; Evaluation of four commercial kits for SARS-CoV-2 real-time reverse-transcription polymerase chain reaction approved by emergency-use-authorization in Korea. Front Med (Lausanne). 7:521. DOI: 10.3389/fmed.2020.00521. PMID: 32903503. PMCID: PMC7438443.7. World Health Organization (WHO). 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. WHO;Geneva: p. 1–7.8. Park J, Kim SY, Lee J, Hong KH. 2023; Clinical evaluation of BioFire COVID-19 test, BioFire respiratory panel 2.1, and Cepheid Xpert Xpress SARS-CoV-2 assays for sample-to-answer detection of SARS-CoV-2. Genes (Basel). 14:233. DOI: 10.3390/genes14010233. PMID: 36672974. PMCID: PMC9859140.9. Baron A, Peyrony O, Salmona M, Mahjoub N, Ellouze S, Anastassiou M, et al. 2022; Impact of fast SARS-CoV-2 molecular point-of-care testing on patients' length of stay in an emergency department. Microbiol Spectr. 10:e0063622. DOI: 10.1128/spectrum.00636-22. PMID: 35730967. PMCID: PMC9431206.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Response to positive patients with COVID-19 self-test visiting the emergency department
- An Experience of Operating the Pediatric Emergency Room
- The impact of the COVID-19 pandemic on in-hospital mortality in patients admitted through the emergency department
- Effect of regional COVID-19 outbreak to emergency department response on acute myocardial infarction: a multicenter retrospective study
- Retrospective Validation of Xpert Xpress SARSCoV-2 and Rapid COVID-19 PCR Tests