Korean J healthc assoc Infect Control Prev.
2024 Jun;29(1):10-18. 10.14192/kjicp.2024.29.1.10.
Advancements and Interpretations of Laboratory Testing for Infectious Pneumonia
- Affiliations
-
- 1Department of Laboratory Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, Kyung Hee University Hospital, Kyung Hee University College of Medicine, Seoul, Korea
- KMID: 2556869
- DOI: http://doi.org/10.14192/kjicp.2024.29.1.10
Abstract
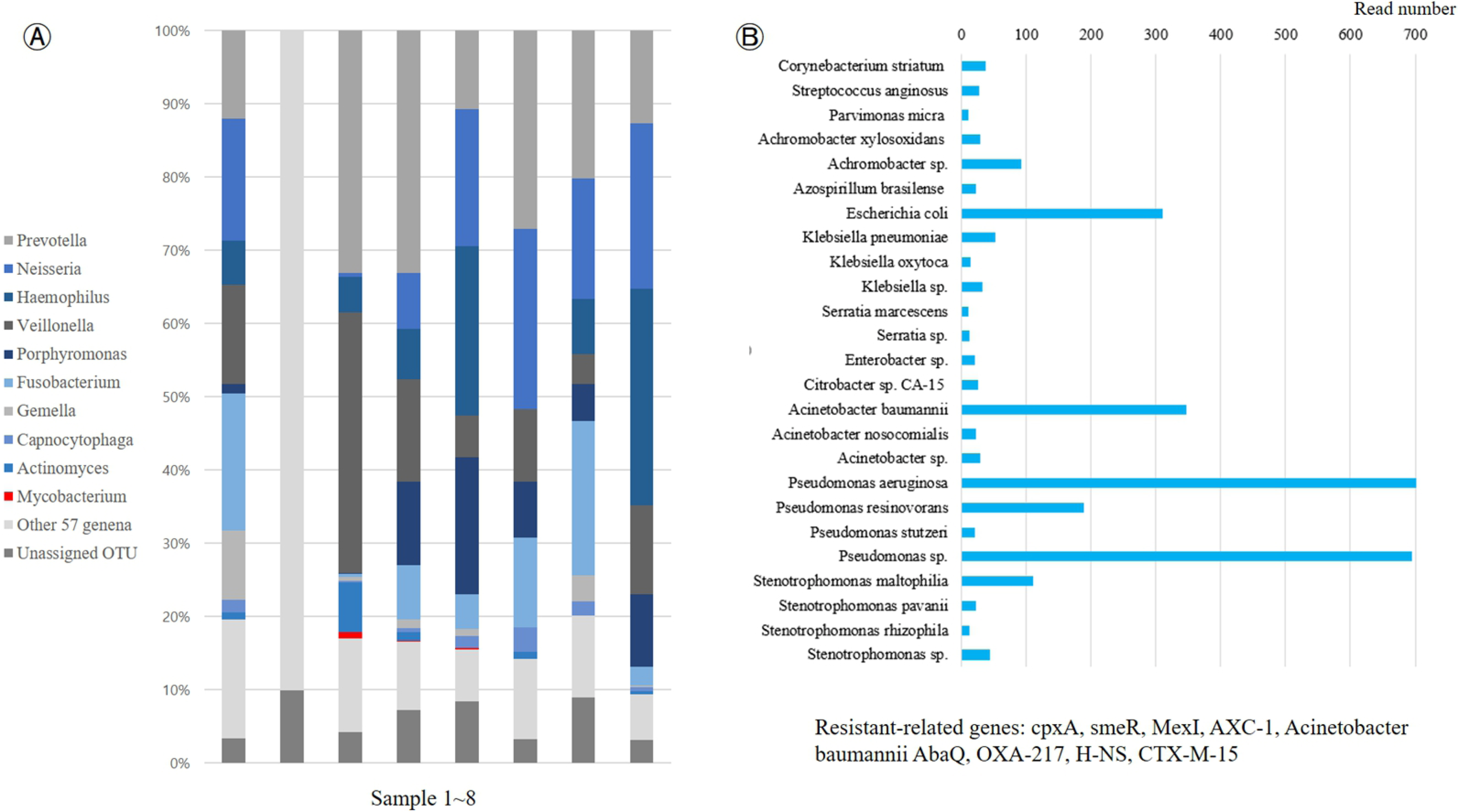
- Respiratory infections pose significant global public health challenges, with pneumonia being the major contributor. Rapid and accurate diagnoses of respiratory infections remain paramount. This review focuses on the evolution of diagnostic laboratory testing for infectious pneumonia and traces advancements from the past to the present. From early Gram staining to the advent of modern molecular biology techniques, various diagnostic approaches are explored. Additionally, the potential advantages, limitations, and practical applications of emerging diagnostic laboratory technologies are discussed with the aim of providing valuable insights for healthcare workers involved in infection control, enhancing their understanding of the latest trends and precise interpretations of pneumonia diagnoses.
Keyword
Figure
Reference
-
1. Mandell LA, Niederman MS. Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson J, editors. 2022. Pneumonia. Harrison's principles of internal medicine. 21st ed. McGraw-Hill Education;New York: p. 1009–19.2. Lanks CW, Musani AI, Hsia DW. 2019; Community-acquired pneumonia and hospital-acquired pneumonia. Med Clin North Am. 103:487–501. DOI: 10.1016/j.mcna.2018.12.008. PMID: 30955516.3. Song JH, Huh K, Chung DR. 2019; Modern history of tuberculosis in Korea. Infect Chemother. 51:414–26. Erratum in: Infect Chemother 2020;52:305-6. DOI: 10.3947/ic.2019.51.4.414. PMID: 31782276. PMCID: PMC6940379.4. Basarab M, Macrae MB, Curtis CM. 2014; Atypical pneumonia. Curr Opin Pulm Med. 20:247–51. DOI: 10.1097/MCP.0000000000000048. PMID: 24626238.5. Lee M. Korean Society for Laboratory Medicine. 2021. Diagnosis of organ-specific bacterial infections. Laboratory medicine. 6th ed. Panmuneducation;Seoul: p. 682–6.6. Pagliano P, Sellitto C, Conti V, Ascione T, Esposito S. 2021; Characteristics of viral pneumonia in the COVID-19 era: an update. Infection. 49:607–16. DOI: 10.1007/s15010-021-01603-y. PMID: 33782861. PMCID: PMC8006879.7. Godoy MCB, Ferreira Dalla Pria HR, Truong MT, Shroff GS, Marom EM. 2022; Invasive fungal pneumonia in immunocompromised patients. Radiol Clin North Am. 60:497–506. DOI: 10.1016/j.rcl.2022.01.006. PMID: 35534133.8. Zlojtro M, Jankovic M, Samarzija M, Zmak L, Jankovic VK, Obrovac M, et al. 2015; Nosocomial pseudo-outbreak of Mycobacterium gordonae associated with a hospital's water supply contamination: a case series of 135 patients. J Water Health. 13:125–30. DOI: 10.2166/wh.2014.061. PMID: 25719472.9. Rattani S, Farooqi J, Jabeen G, Chandio S, Kash Q, Khan A, et al. 2020; Evaluation of semi-quantitative compared to quantitative cultures of tracheal aspirates for the yield of culturable respiratory pathogens - a cross-sectional study. BMC Pulm Med. 20:284. DOI: 10.1186/s12890-020-01311-7. PMID: 33121470. PMCID: PMC7594958.10. Shen L, Wang F, Shi J, Xu W, Jiang T, Tang H, et al. 2019; Microbiological analysis of endotracheal aspirate and endotracheal tube cultures in mechanically ventilated patients. BMC Pulm Med. 19:162. DOI: 10.1186/s12890-019-0926-3. PMID: 31455270. PMCID: PMC6712863.11. Murdoch DR, Morpeth SC, Hammitt LL, Driscoll AJ, Watson NL, Baggett HC, et al. 2017; Microscopic analysis and quality assessment of induced sputum from children with pneumonia in the PERCH study. Clin Infect Dis. 64(suppl_3):S271–9.12. Ogawa H, Kitsios GD, Iwata M, Terasawa T. 2020; Sputum Gram stain for bacterial pathogen diagnosis in community-acquired pneumonia: a systematic review and Bayesian meta-analysis of diagnostic accuracy and yield. Clin Infect Dis. 71:499–513. DOI: 10.1093/cid/ciz876. PMID: 31504334. PMCID: PMC7384319.13. Prinzi AM, Wattier RL, Curtis DJ, Ziniel SI, Fitzgerald A, Pearce K, et al. 2022; Impact of organism reporting from endotracheal aspirate cultures on antimicrobial prescribing practices in mechanically ventilated pediatric patients. J Clin Microbiol. 60:e0093022. DOI: 10.1128/jcm.00930-22. PMID: 36218349. PMCID: PMC9667758.14. Alby K, Smedberg J. Leber AL and Burnham CAD. 2023. Lower respiratory tract cultures. Clinical microbiology procedures handbook. 5th ed. Vol. 1:ASM Press;Washington, DC: 3.10.2.2.15. Klein M, Bacher J, Barth S, Atrzadeh F, Siebenhaller K, Ferreira I, et al. 2021; Multicenter evaluation of the Unyvero platform for testing bronchoalveolar lavage fluid. J Clin Microbiol. 59:e02497–20. DOI: 10.1128/JCM.02497-20. PMID: 33328178. PMCID: PMC8106704.16. Lancefield RC. 1933; A serological differentiation of human and other groups of hemolytic streptococci. J Exp Med. 57:571–95. DOI: 10.1084/jem.57.4.571. PMID: 19870148. PMCID: PMC2132252.17. Facklam R. 2002; What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. 15:613–30. DOI: 10.1128/CMR.15.4.613-630.2002. PMID: 12364372. PMCID: PMC126867.18. Evans TJ, Riley PA. 2021; Principles of microscopy, culture and serology-based diagnostics. Medicine. 49:648–53. DOI: 10.1016/j.mpmed.2021.07.009.19. Croxatto A, Prod'hom G, Greub G. 2012; Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol Rev. 36:380–407. DOI: 10.1111/j.1574-6976.2011.00298.x. PMID: 22092265.20. Šedo O, Nemec A, Křížová L, Kačalová M, Zdráhal Z. 2013; Improvement of MALDI-TOF MS profiling for the differentiation of species within the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. Syst Appl Microbiol. 36:572–8. DOI: 10.1016/j.syapm.2013.08.001. PMID: 24054697.21. Jeong S, Hong JS, Kim JO, Kim KH, Lee W, Bae IK, et al. 2016; Identification of Acinetobacter species using matrix-assisted laser desorption ionization-time of flight mass spectrometry. Ann Lab Med. 36:325–34. DOI: 10.3343/alm.2016.36.4.325. PMID: 27139605. PMCID: PMC4855052.22. Gordon J, Michel G. 2012; Discerning trends in multiplex immunoassay technology with potential for resource-limited settings. Clin Chem. 58:690–8. DOI: 10.1373/clinchem.2011.176503. PMID: 22461515.23. Kim SK, Sung H, Hwang SH, Kim MN. 2022; A new quantum dot-based lateral flow immunoassay for the rapid detection of influenza viruses. Biochip J. 16:175–82. DOI: 10.1007/s13206-022-00053-4. PMID: 35340454. PMCID: PMC8935104.24. Wang C, Yang X, Zheng S, Cheng X, Xiao R, Li Q, et al. 2021; Development of an ultrasensitive fluorescent immunochromatographic assay based on multilayer quantum dot nanobead for simultaneous detection of SARS-CoV-2 antigen and influenza A virus. Sens Actuators B Chem. 345:130372. DOI: 10.1016/j.snb.2021.130372. PMID: 34219970. PMCID: PMC8239248.25. Viasus D, Calatayud L, McBrown MV, Ardanuy C, Carratalà J. 2019; Urinary antigen testing in community-acquired pneumonia in adults: an update. Expert Rev Anti Infect Ther. 17:107–15. DOI: 10.1080/14787210.2019.1565994. PMID: 30618315.26. Parte AC. 2018; LPSN - LIST of Prokaryotic names with Standing in Nomenclature (bacterio.net), 20 years on. Int J Syst Evol Microbiol. 68:1825–9. DOI: 10.1099/ijsem.0.002786. PMID: 29724269.27. Cunha BA, Burillo A, Bouza E. 2016; Legionnaires' disease. Lancet. 387:376–85. DOI: 10.1016/S0140-6736(15)60078-2. PMID: 26231463.28. McMullen CKM, Dougherty B, Medeiros DT, Yasvinski G, Sharma D, Thomas MK. 2024; Estimating the burden of illness caused by domestic waterborne Legionnaires' disease in Canada: 2015-2019. Epidemiol Infect. 152:e18. DOI: 10.1017/S0950268824000013. PMID: 38204334. PMCID: PMC10894893.29. Lee J, Kim D, Hwang KJ, Yoo J, Chun JH, Jung SO. 2021; Investigation of the distribution of antibodies to Legionella pneumophila from suspected Legionellosis, South Korea, 2018-2020. Public Health Wkly Rep. 14:2223–8.30. Guerrero C, Toldos CM, Yagüe G, Ramírez C, Rodríguez T, Segovia M. 2004; Comparison of diagnostic sensitivities of three assays (Bartels enzyme immunoassay [EIA], Biotest EIA, and Binax NOW immunochromatographic test) for detection of Legionella pneumophila serogroup 1 antigen in urine. J Clin Microbiol. 42:467–8. DOI: 10.1128/JCM.42.1.467-468.2004. PMID: 14715807. PMCID: PMC321653.31. Ito A, Yamamoto Y, Ishii Y, Okazaki A, Ishiura Y, Kawagishi Y, et al. 2021; Evaluation of a novel urinary antigen test kit for diagnosing Legionella pneumonia. Int J Infect Dis. 103:42–7. DOI: 10.1016/j.ijid.2020.10.106. PMID: 33176204.32. Sinclair A, Xie X, Teltscher M, Dendukuri N. 2013; Systematic review and meta-analysis of a urine-based pneumococcal antigen test for diagnosis of community-acquired pneumonia caused by Streptococcus pneumoniae. J Clin Microbiol. 51:2303–10. DOI: 10.1128/JCM.00137-13. PMID: 23678060. PMCID: PMC3697702.33. Rueda ZV, Aguilar Y, Maya MA, López L, Restrepo A, Garcés C, et al. 2022; Etiology and the challenge of diagnostic testing of community-acquired pneumonia in children and adolescents. BMC Pediatr. 22:169. DOI: 10.1186/s12887-022-03235-z. PMID: 35361166. PMCID: PMC8968093.34. Choe YJ, Han MS, Choi YY, Sohn YJ, Kim YK, Kim KM, et al. 2021; Trend change of nasopharyngeal colonization with Streptococcus pneumoniae and non-typeable Haemophilus influenzae in children attending daycare centres: nationwide population-based study, South Korea 2014 and 2019. Int J Infect Dis. 111:328–32. DOI: 10.1016/j.ijid.2021.08.065. PMID: 34508859.35. Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. 2011; Viral pneumonia. Lancet. 377:1264–75. DOI: 10.1016/S0140-6736(10)61459-6. PMID: 21435708.36. Korea Disease Control. 2022. 2022 pathogen biosafety information sheet (section 2·3·4 risk groups). Korea Disease Control and Prevention Agency;Cheongju: p. 181–336.37. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. 2019; Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 200:e45–67. DOI: 10.1164/rccm.201908-1581ST. PMID: 31573350. PMCID: PMC6812437.38. Monard C, Pehlivan J, Auger G, Alviset S, Tran Dinh A, Duquaire P, et al. 2020; Multicenter evaluation of a syndromic rapid multiplex PCR test for early adaptation of antimicrobial therapy in adult patients with pneumonia. Crit Care. 24:434. DOI: 10.1186/s13054-020-03114-y. PMID: 32665030. PMCID: PMC7359443.39. Miyashita N. 2022; Atypical pneumonia: pathophysiology, diagnosis, and treatment. Respir Investig. 60:56–67. DOI: 10.1016/j.resinv.2021.09.009. PMID: 34750083.40. Park S, Zhang Y, Lin S, Wang TH, Yang S. 2011; Advances in microfluidic PCR for point-of-care infectious disease diagnostics. Biotechnol Adv. 29:830–9. DOI: 10.1016/j.biotechadv.2011.06.017. PMID: 21741465. PMCID: PMC3186827.41. Dong X, Liu L, Tu Y, Zhang J, Miao G, Zhang L, et al. 2021; Rapid PCR powered by microfluidics: a quick review under the background of COVID-19 pandemic. Trends Analyt Chem. 143:116377. DOI: 10.1016/j.trac.2021.116377. PMID: 34188341. PMCID: PMC8223007.42. Falsey AR, Branche AR, Croft DP, Formica MA, Peasley MR, Walsh EE. 2024; Real-life assessment of BioFire FilmArray Pneumonia panel in adults hospitalized with respiratory illness. J Infect Dis. 229:214–22. DOI: 10.1093/infdis/jiad221. PMID: 37369370. PMCID: PMC10786250.43. Kosai K, Akamatsu N, Ota K, Mitsumoto-Kaseida F, Sakamoto K, Hasegawa H, et al. 2022; BioFire FilmArray Pneumonia Panel enhances detection of pathogens and antimicrobial resistance in lower respiratory tract specimens. Ann Clin Microbiol Antimicrob. 21:24. DOI: 10.1186/s12941-022-00512-8. PMID: 35659683. PMCID: PMC9166201.44. Yoo IY, Seok HS, Kwon JA, Lee J, Jo S, Kim SY, et al. 2023; Evaluation of the BioFire® FilmArray® Pneumonia panel with conventional bacterial culture in conjunction with leukocyte esterase test. Diagnostics (Basel). 13:1847. DOI: 10.3390/diagnostics13111847. PMID: 37296700. PMCID: PMC10252269.45. Gadsby NJ, Russell CD, McHugh MP, Mark H, Conway Morris A, Laurenson IF, et al. 2016; Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis. 62:817–23. DOI: 10.1093/cid/civ1214. PMID: 26747825. PMCID: PMC4787606.46. Miller MM, Van Schooneveld TC, Stohs EJ, Marcelin JR, Alexander BT, Watkins AB, et al. 2023; Implementation of a rapid multiplex polymerase chain reaction pneumonia panel and subsequent antibiotic de-escalation. Open Forum Infect Dis. 10:ofad382. DOI: 10.1093/ofid/ofad382. PMID: 37564742. PMCID: PMC10411041.47. Wils J, Saegeman V, Schuermans A. 2022; Impact of multiplexed respiratory viral panels on infection control measures and antimicrobial stewardship: a review of the literature. Eur J Clin Microbiol Infect Dis. 41:187–202. DOI: 10.1007/s10096-021-04375-3. PMID: 34799754. PMCID: PMC8604699.48. Markussen DL, Serigstad S, Ritz C, Knoop ST, Ebbesen MH, Faurholt-Jepsen D, et al. 2024; Diagnostic stewardship in community-acquired pneumonia with syndromic molecular testing: a randomized clinical trial. JAMA Netw Open. 7:e240830. DOI: 10.1001/jamanetworkopen.2024.0830. PMID: 38446481. PMCID: PMC10918504.49. Slatko BE, Gardner AF, Ausubel FM. 2018; Overview of next-generation sequencing technologies. Curr Protoc Mol Biol. 122:e59. DOI: 10.1002/cpmb.59. PMID: 29851291. PMCID: PMC6020069.50. Deurenberg RH, Bathoorn E, Chlebowicz MA, Couto N, Ferdous M, García-Cobos S, et al. 2017; Application of next generation sequencing in clinical microbiology and infection prevention. J Biotechnol. 243:16–24. DOI: 10.1016/j.jbiotec.2016.12.022. PMID: 28042011.51. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. 2020; A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 382:727–33. DOI: 10.1056/NEJMoa2001017. PMID: 31978945. PMCID: PMC7092803.52. Marchesi JR, Ravel J. 2015; The vocabulary of microbiome research: a proposal. Microbiome. 3:31. DOI: 10.1186/s40168-015-0094-5. PMID: 26229597. PMCID: PMC4520061.53. de Steenhuijsen Piters WA, Huijskens EG, Wyllie AL, Biesbroek G, van den Bergh MR, Veenhoven RH, et al. 2016; Dysbiosis of upper respiratory tract microbiota in elderly pneumonia patients. ISME J. 10:97–108. DOI: 10.1038/ismej.2015.99. PMID: 26151645. PMCID: PMC4681870.54. Cummings LA, Kurosawa K, Hoogestraat DR, SenGupta DJ, Candra F, Doyle M, et al. 2016; Clinical next generation sequencing outperforms standard microbiological culture for characterizing polymicrobial samples. Clin Chem. 62:1465–73. DOI: 10.1373/clinchem.2016.258806. PMID: 27624135.55. Takeuchi S, Kawada JI, Horiba K, Okuno Y, Okumura T, Suzuki T, et al. 2019; Metagenomic analysis using next-generation sequencing of pathogens in bronchoalveolar lavage fluid from pediatric patients with respiratory failure. Sci Rep. 9:12909. DOI: 10.1038/s41598-019-49372-x. PMID: 31501513. PMCID: PMC6733840.56. Hasan MR, Rawat A, Tang P, Jithesh PV, Thomas E, Tan R, et al. 2016; Depletion of human DNA in spiked clinical specimens for improvement of sensitivity of pathogen detection by next-generation sequencing. J Clin Microbiol. 54:919–27. DOI: 10.1128/JCM.03050-15. PMID: 26763966. PMCID: PMC4809942.57. Leo S, Gaïa N, Ruppé E, Emonet S, Girard M, Lazarevic V, et al. 2017; Detection of bacterial pathogens from broncho-alveolar lavage by next-generation sequencing. Int J Mol Sci. 18:2011. DOI: 10.3390/ijms18092011. PMID: 28930150. PMCID: PMC5618659.58. Man WH, de Steenhuijsen Piters WA, Bogaert D. 2017; The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 15:259–70. DOI: 10.1038/nrmicro.2017.14. PMID: 28316330. PMCID: PMC7097736.59. Haak BW, Brands X, Davids M, Peters-Sengers H, Kullberg RFJ, van Houdt R, et al. 2022; Bacterial and viral respiratory tract microbiota and host characteristics in adults with lower respiratory tract infections: a case-control study. Clin Infect Dis. 74:776–84. DOI: 10.1093/cid/ciab568. PMID: 34156449. PMCID: PMC8906706.60. Thorburn F, Bennett S, Modha S, Murdoch D, Gunson R, Murcia PR. 2015; The use of next generation sequencing in the diagnosis and typing of respiratory infections. J Clin Virol. 69:96–100. Erratum in: J Clin Virol 2015;70:128. DOI: 10.1016/j.jcv.2015.06.101. PMID: 28889994. PMCID: PMC5597557.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interpretations of abnormal findings of nonspecific laboratory tests in infectious disease
- Aerosolized antibiotics in the treatment of hospital-acquired pneumonia/ventilator-associated pneumonia
- Interpretation of Papanicolaou Smear Test and Gram Stain Results for the Diagnosis of Infectious Vaginitis is Affected by Knowledge of Additional Related Test Results
- Respiratory Review of 2010: Pneumonia
- Clinical study of mycoplasma pneumoniae pneumonia in children