Kosin Med J.
2024 Mar;39(1):60-65. 10.7180/kmj.23.118.
Primary gastric leiomyosarcoma: a case report and literature review
- Affiliations
-
- 1Department of Radiology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- KMID: 2556799
- DOI: http://doi.org/10.7180/kmj.23.118
Abstract
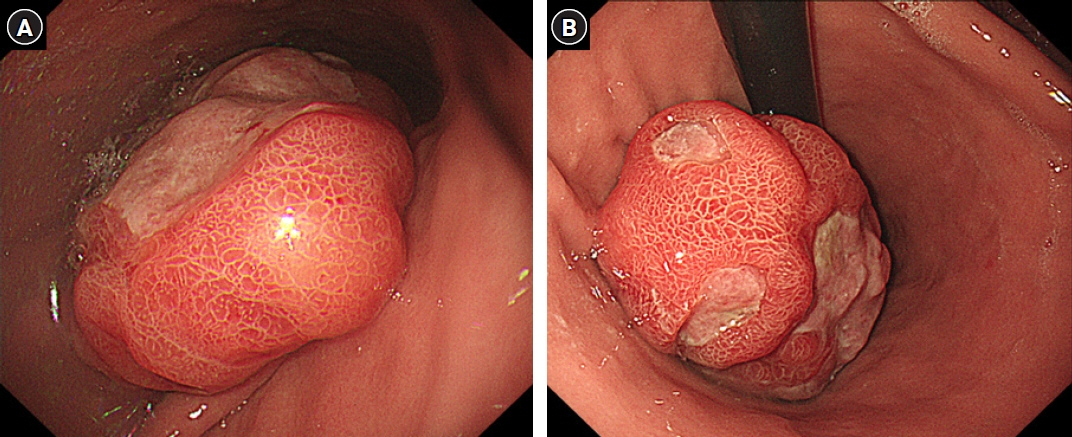
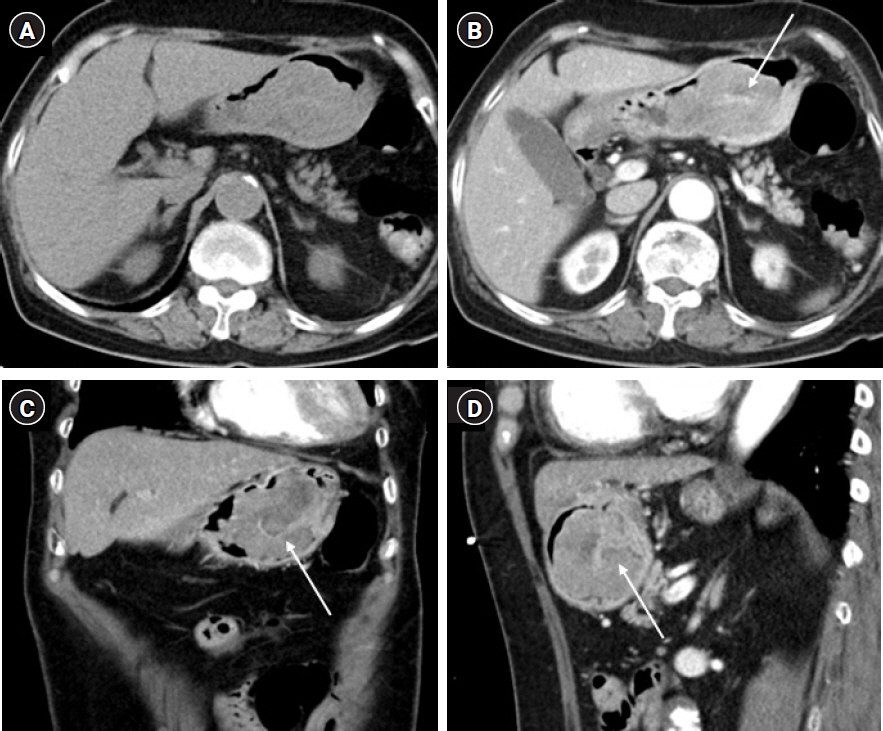
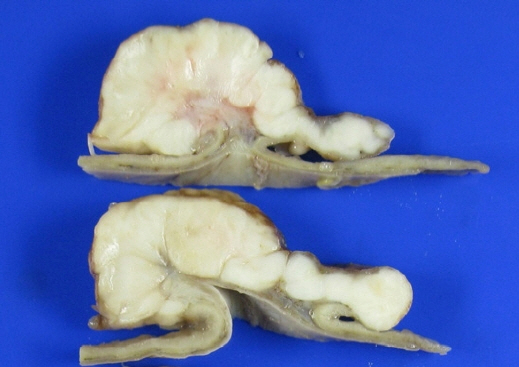
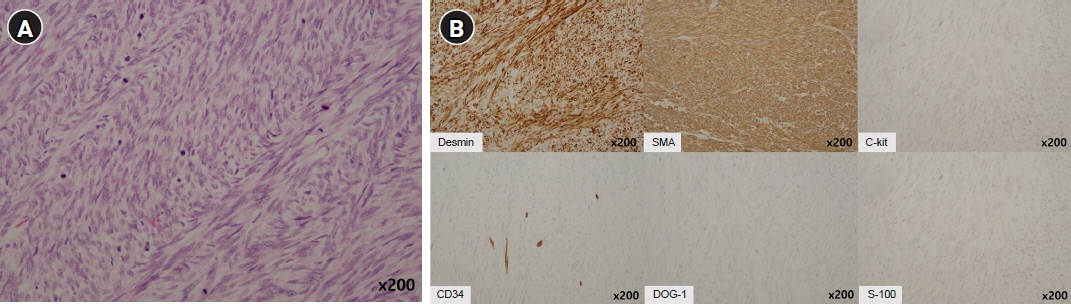
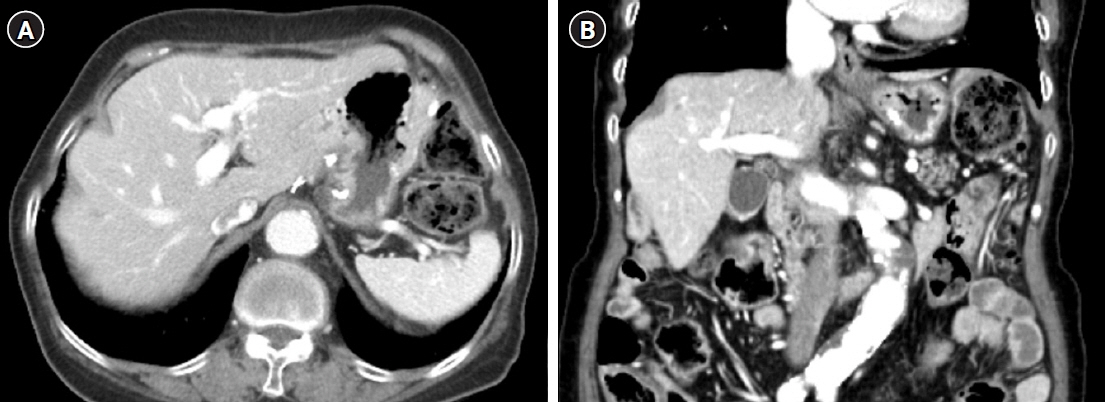
- After separating gastrointestinal (GI) stromal tumors from true smooth muscle tumors of the GI tract, leiomyosarcoma (LMS) of the GI tract has become a rare tumor. Gastric LMS is extremely rare and accounts for 0.1% of all cases of LMS in the GI tract. There are few English-language reports of gastric LMS describing radiologic findings. Here, we report a case of gastric LMS and review the recent literature focusing on radiologic findings. An 80-year-old female patient was referred for evaluation of a gastric mass accompanied by severe anemia. The physical examination revealed no specific findings except for an anemic conjunctiva. Laboratory data showed a low hemoglobin level of 5.1 g/dL. Endoscopy revealed a huge subepithelial mass in the posterior wall of the gastric body. Contrast-enhanced computed tomographic images showed an intraluminal protruding enhancing mass with an internal stalk appearance in the gastric body. There was no internal necrosis or calcification. The patient underwent subtotal gastrectomy and was diagnosed with primary gastric LMS. The diagnosis of gastric LMS is challenging due to its rarity. Our case report suggests that the presence of an internal stalk or spouting appearance can help prompt the radiologist to consider gastric LMS in the differential diagnosis.
Figure
Reference
-
References
1. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998; 279:577–80.2. Agaimy A, Wunsch PH. True smooth muscle neoplasms of the gastrointestinal tract: morphological spectrum and classification in a series of 85 cases from a single institute. Langenbecks Arch Surg. 2007; 392:75–81.3. Yamamoto H, Handa M, Tobo T, Setsu N, Fujita K, Oshiro Y, et al. Clinicopathological features of primary leiomyosarcoma of the gastrointestinal tract following recognition of gastrointestinal stromal tumours. Histopathology. 2013; 63:194–207.4. Biswas M, Rahi R, Tiwary SK, Khanna AK, Khanna R. Leiomyosarcoma of stomach: a case report. Kathmandu Univ Med J (KUMJ). 2006; 4:510–2.5. Miettinen M, Fetsch JF. Evaluation of biological potential of smooth muscle tumours. Histopathology. 2006; 48:97–105.6. Ayoola EA, Arab MM, Tadros NM, Ahmed MA, Banzal SS, Abbo HM, et al. Multicentric leiomyosarcoma of the stomach. Saudi J Gastroenterol. 2003; 9:79–81.7. Insabato L, Di Vizio D, Ciancia G, Pettinato G, Tornillo L, Terracciano L. Malignant gastrointestinal leiomyosarcoma and gastrointestinal stromal tumor with prominent osteoclast-like giant cells. Arch Pathol Lab Med. 2004; 128:440–3.8. Pauser U, Grimm H. Intramucosal leiomyosarcoma of the stomach following hereditary retinoblastoma in childhood: a case report and review of the literature. World J Surg Oncol. 2008; 6:131.9. Soufi M, Errougani A, Chekkof RM. Primary gastric leiomyosarcoma in young revealed by a massive hematemesis. J Gastrointest Cancer. 2009; 40:69–72.10. Masuzawa N, Kishimoto M, Nishimura A, Ichiba N, Aoki E, Yanagibashi K, et al. Gastric leiomyosarcoma manifesting peculiar findings: radiological-pathological correlation. Pathol Int. 2009; 59:306–11.11. Aggarwal G, Sharma S, Zheng M, Reid MD, Crosby JH, Chamberlain SM, et al. Primary leiomyosarcomas of the gastrointestinal tract in the post-gastrointestinal stromal tumor era. Ann Diagn Pathol. 2012; 16:532–40.12. Rou WS, Ju JS, Kang SH, Moon HS, Sung JK, Lee BS, et al. A case of gastric leiomyosarcoma with multiple metastases. Korean J Gastroenterol. 2015; 65:112–7.13. Hilal L, Barada K, Mukherji D, Temraz S, Shamseddine A. Gastrointestinal (GI) leiomyosarcoma (LMS) case series and review on diagnosis, management, and prognosis. Med Oncol. 2016; 33:20.14. Mehta V, Rajawat M, Rastogi S, Phulware RH, Mezencev R. Leiomyosarcoma of the stomach with metastasis to the liver: a case report with review of the literature. Future Sci OA. 2017; 4:FSO264.15. Sato T, Akahoshi K, Tomoeda N, Kinoshita N, Kubokawa M, Yodoe K, et al. Leiomyosarcoma of the stomach treated by endoscopic submucosal dissection. Clin J Gastroenterol. 2018; 11:291–6.16. Hasnaoui A, Jouini R, Haddad D, Zaafouri H, Bouhafa A, Ben Maamer A, et al. Gastric leiomyosarcoma and diagnostic pitfalls: a case report. BMC Surg. 2018; 18:62.17. Kang WZ, Xue LY, Tian YT. Leiomyosarcoma of the stomach: a case report. World J Clin Cases. 2019; 7:3575–82.18. Garg R, AlRajjal A, Berri R, Barawi M. Primary gastric leiomyosarcoma: a case report and review of the literature. J Gastrointest Cancer. 2020; 51:335–40.19. Gubatan J, Shah N. Gastric leiomyosarcoma unmasked by bleeding from a percutaneous endoscopic gastrostomy tube. ACG Case Rep J. 2020; 7:e00301.20. Al-Yousofy F, Alshargabi G, Ahmed F, Almohtadi A, Fazea M, Altam A. Primary huge gastric leiomyosarcoma with multiple metastases in a 60-year-old female: a case report. Pan Afr Med J. 2022; 42:223.