J Korean Neurosurg Soc.
2024 Jul;67(4):442-450. 10.3340/jkns.2023.0183.
Early Detection of hyperemia with Magnetic Resonance Fluid Attenuation Inversion Recovery Imaging after Superficial Temporal Artery to Middle Cerebral Artery Anastomosis
- Affiliations
-
- 1Department of Neurosurgery, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Neurosurgery, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2556741
- DOI: http://doi.org/10.3340/jkns.2023.0183
Abstract
Objective
: Cerebral hyperperfusion syndrome (CHS) manifests as a collection of symptoms brought on by heightened focal cerebral blood flow (CBF), afflicting nearly 30% of patients who have undergone superficial temporal artery (STA)-middle cerebral artery (MCA) anastomosis. The aim of this study was to investigate whether the amalgamation of magnetic resonance imaging (MRI) fluid-attenuated inversion recovery (FLAIR) and apparent diffusion coefficient (ADC) imaging via MRI can discern cerebral hyperemia after STA-MCA anastomosis surgery.
Methods
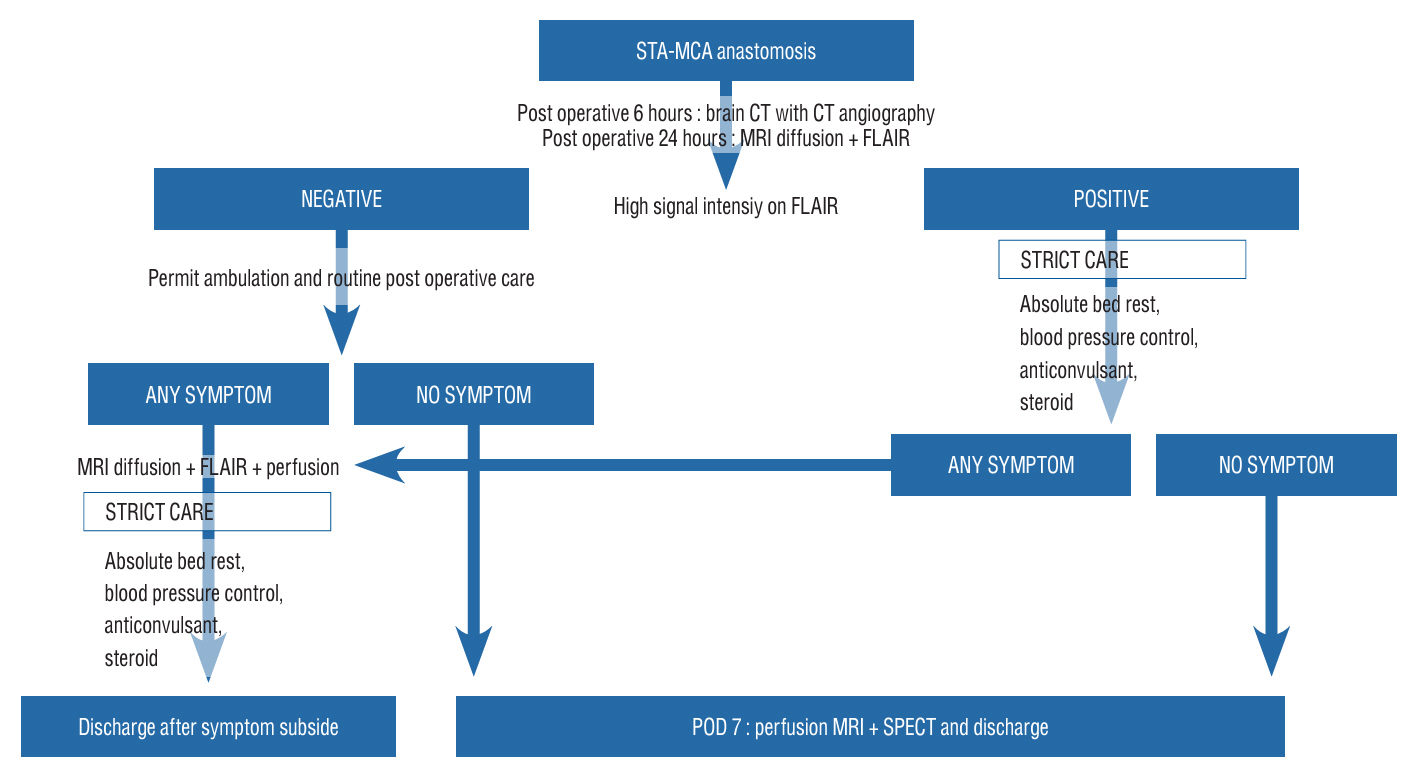
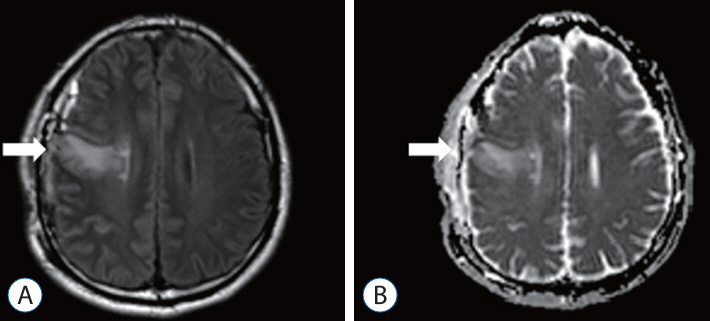
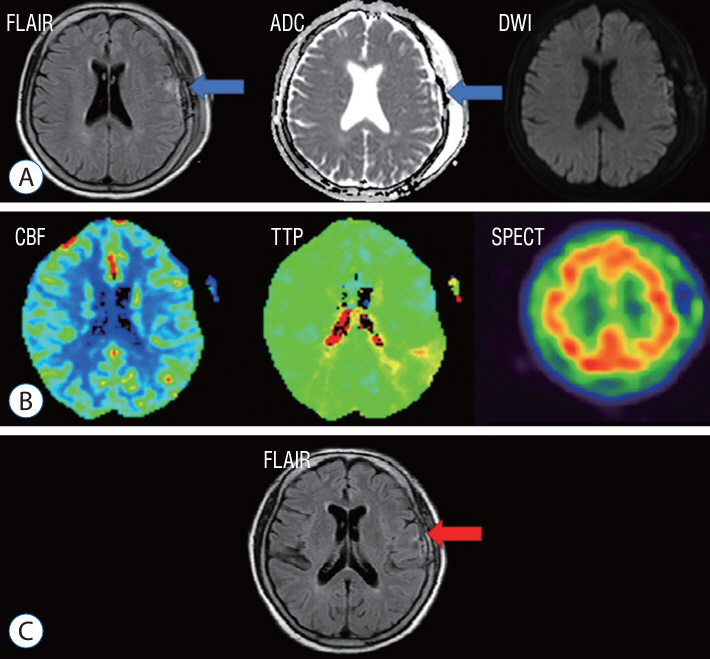
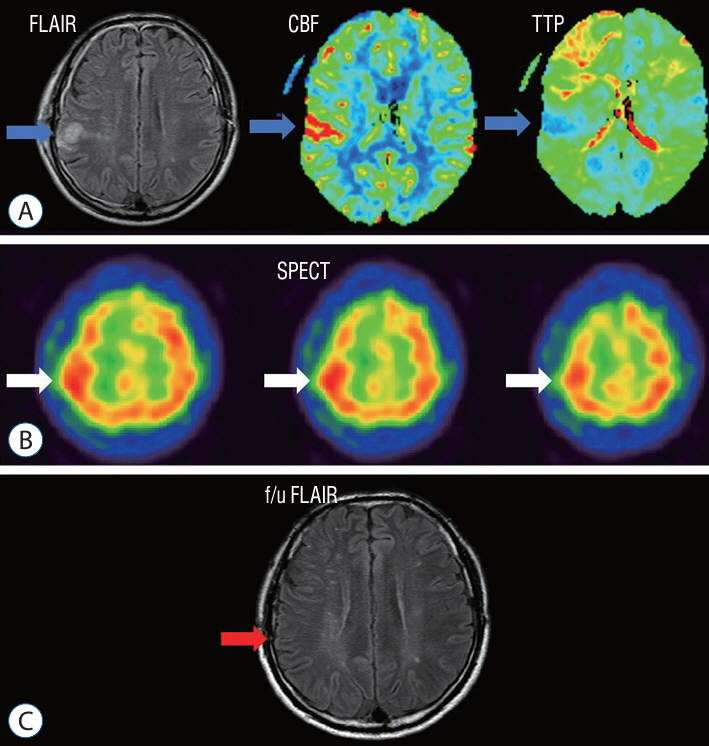
: A retrospective study was performed of patients who underwent STA-MCA anastomosis due to Moyamoya disease or atherosclerotic steno-occlusive disease. A protocol aimed at preventing CHS was instituted, leveraging the use of MRI FLAIR. Patients underwent MRI diffusion with FLAIR imaging 24 hours after STA-MCA anastomosis. A high signal on FLAIR images signified the presence of hyperemia at the bypass site, triggering a protocol of hyperemia care. All patients underwent hemodynamic evaluations, including perfusion MRI, single-photon emission computed tomography (SPECT), and digital subtraction angiography, both before and after the surgery. If a high signal intensity is observed on MRI FLAIR within 24 hours of the surgery, a repeat MRI is performed to confirm the presence of hyperemia. Patients with confirmed hyperemia are managed according to a protocol aimed at preventing further progression.
Results
: Out of a total of 162 patients, 24 individuals (comprising 16 women and 8 men) exhibited hyperemia on their MRI FLAIR scans following the procedure. SPECT was conducted on 23 patients, and 11 of them yielded positive results. All 24 patients underwent perfusion MRI, but nine of them showed no significant findings. Among the patients, 10 displayed elevations in both CBF and cerebral blood volume (CBV), three only showed elevation in CBF, and two only showed elevation in CBV. Follow-up MRI FLAIR scans conducted 6 months later on these patients revealed complete normalization of the previously observed high signal intensity, with no evidence of ischemic injury.
Conclusion
: The study determined that the use of MRI FLAIR and ADC mapping is a competent means of early detection of hyperemia after STA-MCA anastomosis surgery. The protocol established can be adopted by other neurosurgical institutions to enhance patient outcomes and mitigate the hazard of permanent cerebral injury caused by cerebral hyperemia.
Figure
Reference
-
References
1. Benzel EC, Hoppens KD. Factors associated with postoperative hypertension complicating carotid endarterectomy. Acta Neurochir (Wien). 112:8–12. 1991.
Article2. Eun J, Park IS. Early restoration of hypoperfusion confirmed by perfusion magnetic resonance image after emergency superficial temporal artery to middle cerebral artery anastomosis. J Korean Neurosurg Soc. 65:816–824. 2022.
Article3. Farooq MU, Goshgarian C, Min J, Gorelick PB. Pathophysiology and management of reperfusion injury and hyperperfusion syndrome after carotid endarterectomy and carotid artery stenting. Exp Transl Stroke Med. 8:7. 2016.
Article4. ujimura M, Inoue T, Shimizu H, Saito A, Mugikura S, Tominaga T. Efficacy of prophylactic blood pressure lowering according to a standardized postoperative management protocol to prevent symptomatic cerebral hyperperfusion after direct revascularization surgery for moyamoya disease. Cerebrovasc Dis. 33:436–445. 2012.
Article5. Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T. Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol. 71:442–447. 2009.
Article6. Fujimura M, Shimizu H, Mugikura S, Tominaga T. Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol. 71:223–227. discussion 227. 2009.
Article7. eschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 161:186–187. 1968.
Article8. Hayashi K, Horie N, Suyama K, Nagata I. Incidence and clinical features of symptomatic cerebral hyperperfusion syndrome after vascular reconstruction. World Neurosurg. 78:447–454. 2012.
Article9. Heros RC, Scott RM, Kistler JP, Ackerman RH, Conner ES. Temporary neurological deterioration after extracranial-intracranial bypass. Neurosurgery. 15:178–185. 1984.
Article10. Janigro D, West GA, Nguyen TS, Winn HR. Regulation of blood-brain barrier endothelial cells by nitric oxide. Circ Res. 75:528–538. 1994.
Article11. Kaku Y, Iihara K, Nakajima N, Kataoka H, Fukuda K, Masuoka J, et al. Cerebral blood flow and metabolism of hyperperfusion after cerebral revascularization in patients with moyamoya disease. J Cereb Blood Flow Metab. 32:2066–2075. 2012.
Article12. Kim JE, Oh CW, Kwon OK, Park SQ, Kim SE, Kim YK. Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis. 25:580–586. 2008.
Article13. Kim JS. Moyamoya disease: epidemiology, clinical features, and diagnosis. J Stroke. 18:2–11. 2016.
Article14. Nomura S, Yamaguchi K, Ishikawa T, Kawashima A, Okada Y, Kawamata T. Factors of delayed hyperperfusion and the importance of repeated cerebral blood flow evaluation for hyperperfusion after direct bypass for Moyamoya disease. World Neurosurg. 118:e468–e472. 2018.
Article15. Ogasawara K, Inoue T, Kobayashi M, Endo H, Fukuda T, Ogawa A. Pretreatment with the free radical scavenger edaravone prevents cerebral hyperperfusion after carotid endarterectomy. Neurosurgery. 55:1060–1067. 2004.
Article16. Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Iihara K, Toyoda K, et al. Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg. 107:1130–1136. 2007.
Article17. Ohta T, Tanaka H, Kuroiwa T. Diffuse leptomeningeal enhancement, “ivy sign,” in magnetic resonance images of moyamoya disease in childhood: case report. Neurosurgery. 37:1009–1012. 1995.
Article18. Ohue S, Kumon Y, Kohno K, Watanabe H, Iwata S, Ohnishi T. Postoperative temporary neurological deficits in adults with moyamoya disease. Surg Neurol. 69:281–286. discussion 286-287. 2008.
Article19. Pang CH, Lee SU, Lee Y, Kim WB, Kwon MY, Sunwoo L, et al. Prediction of hemorrhagic cerebral hyperperfusion syndrome after direct bypass surgery in adult nonhemorrhagic moyamoya disease: combining quantitative parameters on RAPID perfusion CT with clinically related factors. J Neurosurg. 138:683–692. 2022.
Article20. Piepgras DG, Morgan MK, Sundt TM Jr, Yanagihara T, Mussman LM. Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg. 68:532–536. 1988.
Article21. Sato K, Yamada M, Kuroda H, Yamamoto D, Asano Y, Inoue Y, et al. Time-of-flight MR angiography for detection of cerebral hyperperfusion syndrome after superficial temporal artery-middle cerebral artery anastomosis in moyamoya disease. AJNR Am J Neuroradiol. 37:1244–1248. 2016.
Article22. Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 4:37–48. 2003.
Article23. Uchino H, Kuroda S, Hirata K, Shiga T, Houkin K, Tamaki N. Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: a serial single photon emission CT/positron emission tomography study. Stroke. 43:2610–2616. 2012.
Article24. Uno M, Nakajima N, Nishi K, Shinno K, Nagahiro S. Hyperperfusion syndrome after extracranial-intracranial bypass in a patient with moyamoya disease--case report. Neurol Med Chir (Tokyo). 38:420–424. 1998.
Article25. van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, et al. Cerebral hyperperfusion syndrome. Lancet Neurol. 4:877–888. 2005.
Article26. Yu J, Zhang J, Li J, Zhang J, Chen J. Cerebral hyperperfusion syndrome after revascularization surgery in patients with moyamoya disease: systematic review and meta-analysis. World Neurosurg. 135:357–366.e4. 2020.
Article27. Zhao WG, Luo Q, Jia JB, Yu JL. Cerebral hyperperfusion syndrome after revascularization surgery in patients with moyamoya disease. Br J Neurosurg. 27:321–325. 2013.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- External Carotid Artery Angioplasty and Stenting Followed by Superficial Temporal Artery to Middle Cerebral Artery Anastomosis
- A Middle Cerebral Artery AneurysmOriginating Near the Site of Anastomosis after Superficial Temporal Artery-Middle Cerebral Artery Bypass: Case Report
- Superficial Temporal Artery-Middle Cerebral Artery Anastomosis for Internal Carotid Artery Occlusion by Subacute In-Stent Thrombosis after Carotid Artery Stenting
- The Usefulness of the Ivy Sign on Fluid-Attenuated Intensity Recovery Images in Improved Brain Hemodynamic Changes after Superficial Temporal Artery-Middle Cerebral Artery Anastomosis in Adult Patients with Moyamoya Disease
- Hyperintense Vessels on FLAIR MRI in Patients With Acute Middle Cerebral Artery Infarction Revealed Pial Collateral on Cerebral Angiography