J Neurocrit Care.
2024 Jun;17(1):7-15. 10.18700/jnc.240016.
Non-invasive and continuous monitoring of cerebral blood flow as a parameter for neurological deterioration in acute brain injury
- Affiliations
-
- 1Department of Neurology, Soonchunhyang University Hospital Seoul, Seoul, Korea
- 2Department of Neurology, Seoul National University Hospital, Seoul, Korea
- 3Department of Critical Care Medicine, Seoul National University Hospital, Seoul, Korea
- 4Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea
- KMID: 2556728
- DOI: http://doi.org/10.18700/jnc.240016
Abstract
- Background
Monitoring the cerebral blood flow (CBF) is crucial when caring for patients in neurological intensive care units (NICU). Changes in CBF, either due to hypo- or hyperperfusion, have been associated with neurological deterioration. By using a non-invasive continuous CBF monitor, we aimed to assess whether cerebral flow index (CFI) fluctuations could correlate with neurological deterioration.
Methods
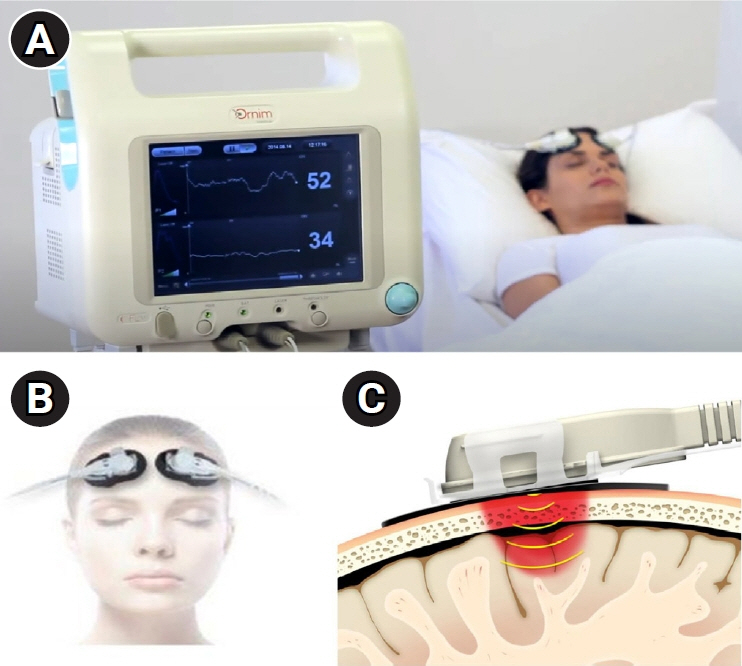
We prospectively collected data from patients with acute brain injury (subarachnoid hemorrhage [SAH], Moyamoya disease [MMD], and ischemic stroke), who were at a high risk for CBF disturbance between May 2017 and June 2019. Non-invasive CBF measurements were performed in the bilateral prefrontal cortex using a c-FLOW device. Continuous CBF was assessed using CFI. The delta value and percent change in the CFI were compared between patients with and without neurological deterioration.
Results
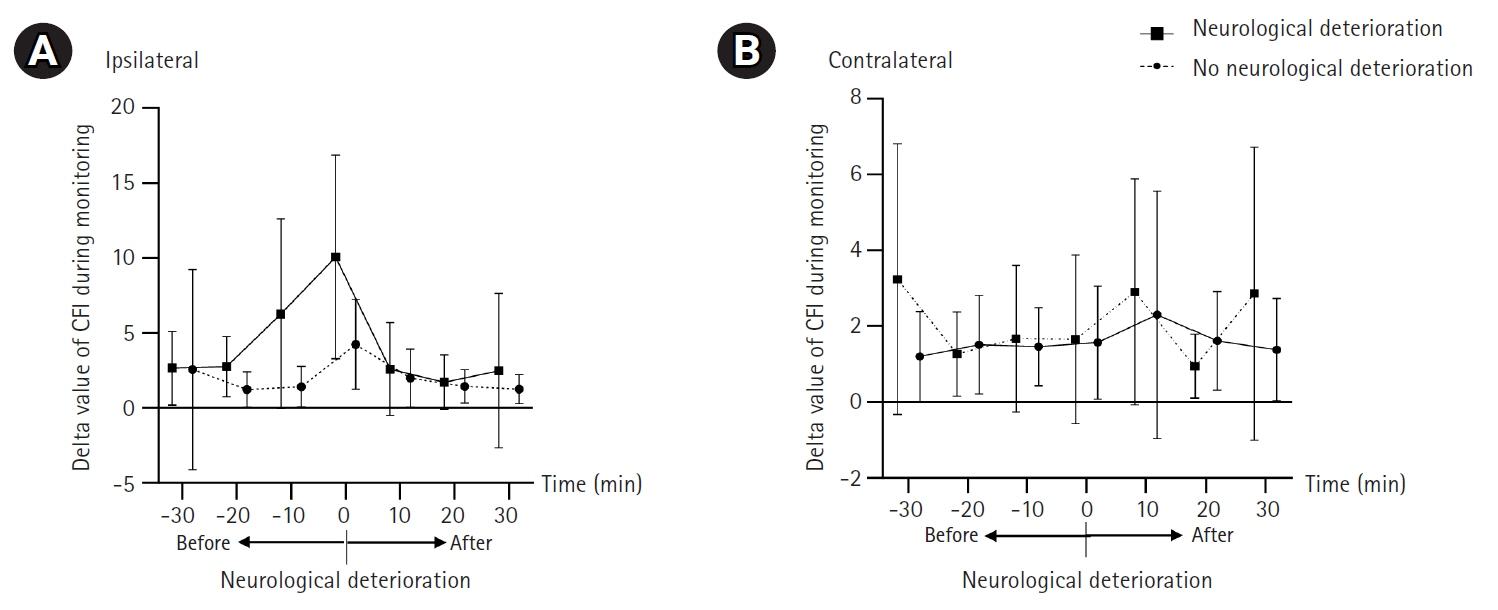
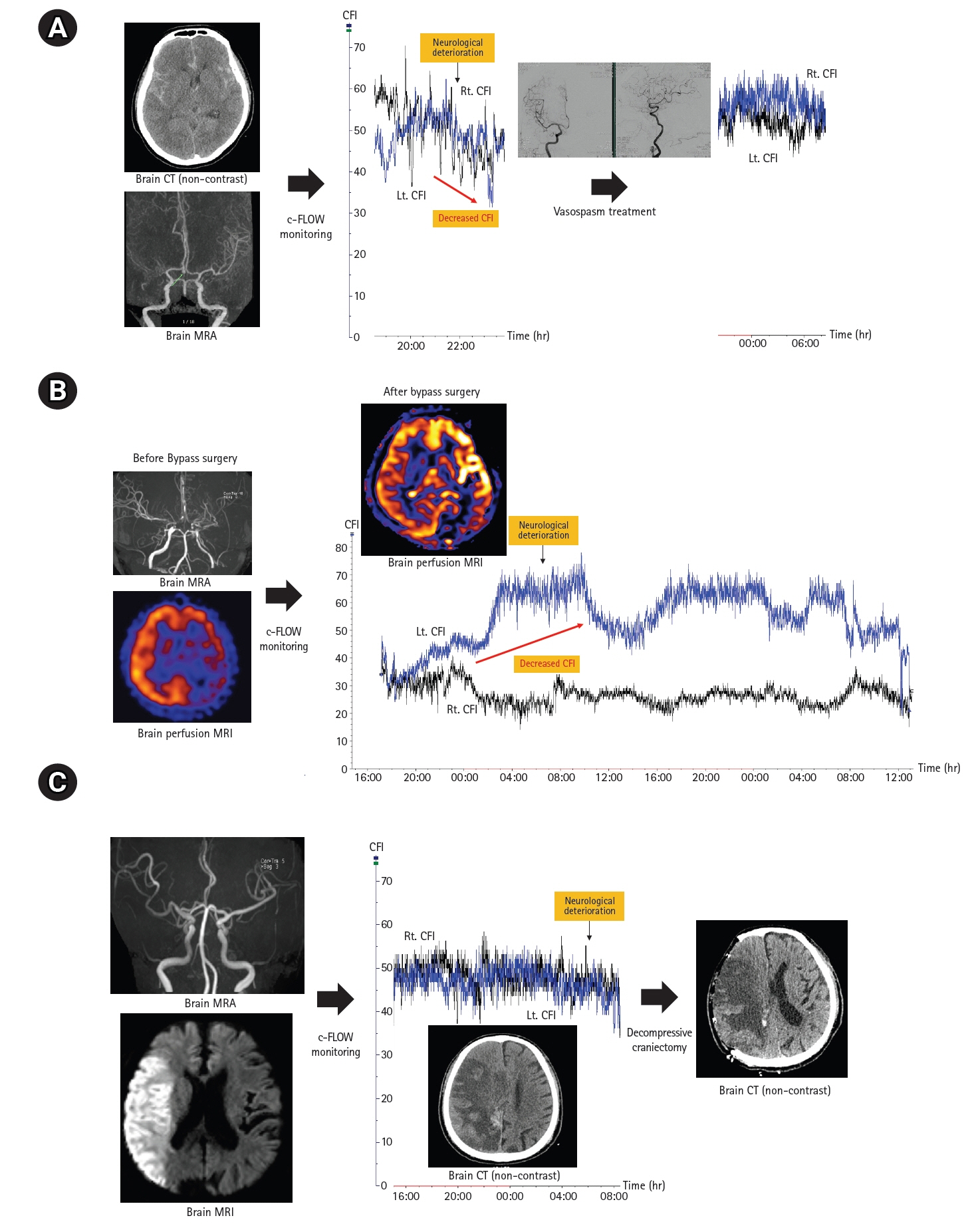
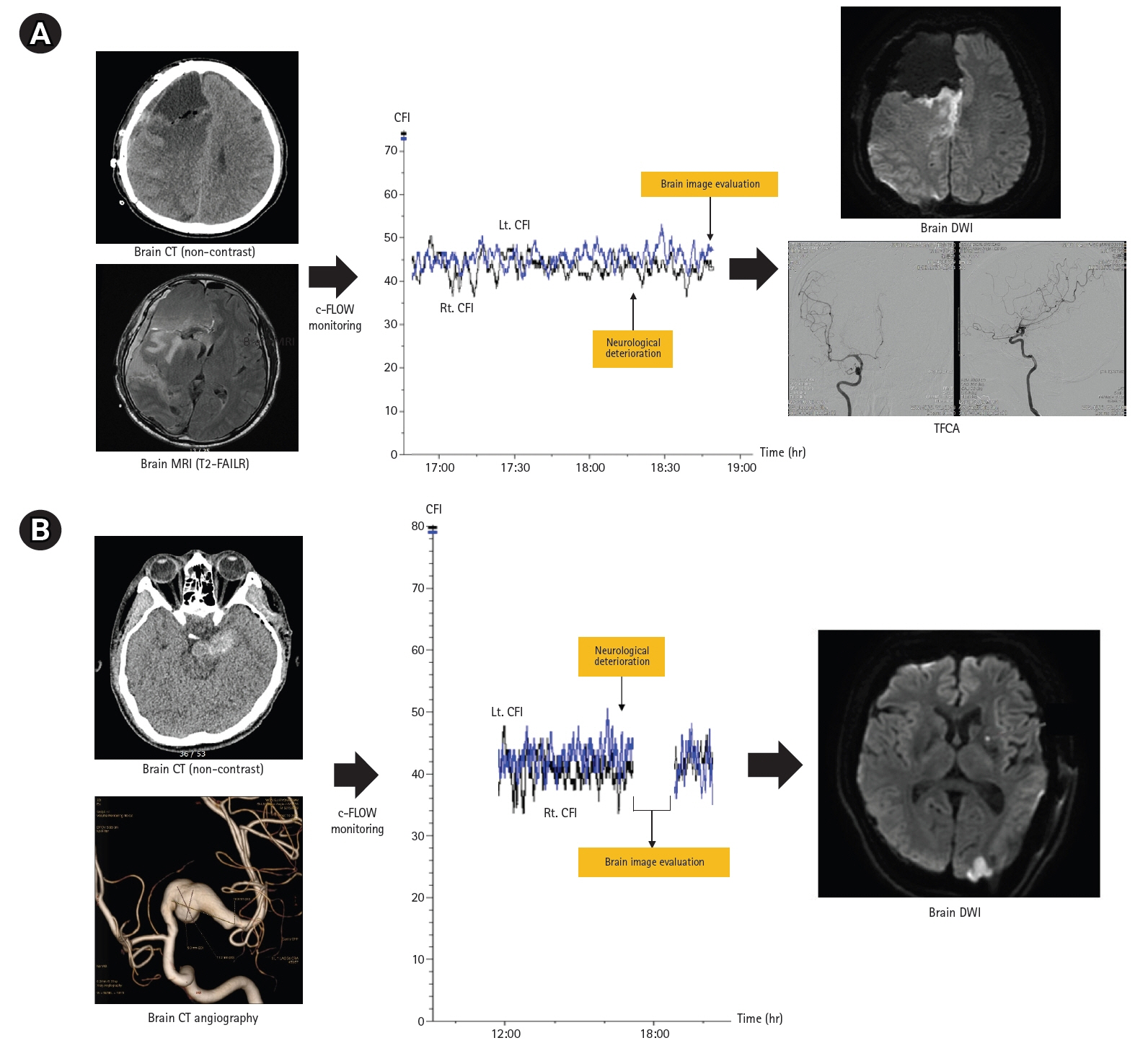
A total of 45 patients (mean age, 51.6 years; male, 48.9%) were included in our analysis (SAH, 13; MMD, 17; ischemic stroke,15). The mean monitoring duration was approximately 52 hours. Nine patients (20.0%) had neurologic worsening during c-FLOW monitoring in NICU. The delta value (median, 10.4; interquartile range [IQR], 3.9–14.3 vs. median, 3.4; IQR, 2.5–5.6; P=0.008) and percent change in CFI (28.5% vs. 9.0%, P<0.001) was significantly higher in groups with neurological deterioration. In two patients with neurological deterioration, no CFI change was observed because aggravation of cerebral perfusion occurred outside the area of CFI monitoring.
Conclusion
Continuous non-invasive CBF monitoring with c-FLOW may be useful for patients with acute brain injury at high risk for CBF alterations.
Figure
Reference
-
1. Meyer JS, Gotoh F, Akiyama M, Yoshitake S. Monitoring cerebral blood flow, oxygen, and glucose metabolism: analysis of cerebral metabolic disorder in stroke and some therapeutic trials in human volunteers. Circulation. 1967; 36:197–211.2. Mori S, Sadoshima S, Fujii K, Ibayashi S, Iino K, Fujishima M. Decrease in cerebral blood flow with blood pressure reductions in patients with chronic stroke. Stroke. 1993; 24:1376–81.3. Soustiel JF, Glenn TC, Vespa P, Rinsky B, Hanuscin C, Martin NA. Assessment of cerebral blood flow by means of blood-flow-volume measurement in the internal carotid artery: comparative study with a 133xenon clearance technique. Stroke. 2003; 34:1876–80.4. Rubin G, Firlik AD, Levy EI, Pindzola RR, Yonas H. Relationship between cerebral blood flow and clinical outcome in acute stroke. Cerebrovasc Dis. 2000; 10:298–306.5. Kelly DF, Martin NA, Kordestani R, Counelis G, Hovda DA, Bergsneider M, et al. Cerebral blood flow as a predictor of outcome following traumatic brain injury. J Neurosurg. 1997; 86:633–41.6. Wang Q, Miao P, Modi HR, Garikapati S, Koehler RC, Thakor NV. Therapeutic hypothermia promotes cerebral blood flow recovery and brain homeostasis after resuscitation from cardiac arrest in a rat model. J Cereb Blood Flow Metab. 2019; 39:1961–73.7. Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002; 30:733–8.8. Yousef KM, Balzer JR, Bender CM, Hoffman LA, Poloyac SM, Ye F, et al. Cerebral perfusion pressure and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Am J Crit Care. 2015; 24:e65–71.9. Obrist WD, Gennarelli TA, Segawa H, Dolinskas CA, Langfitt TW. Relation of cerebral blood flow to neurological status and outcome in head-injured patients. J Neurosurg. 1979; 51:292–300.10. Sethuraman M. Cerebral blood flow monitoring. J Neuroanaesth Crit Care. 2015; 2:204–14.11. Donnelly J, Budohoski KP, Smielewski P, Czosnyka M. Regulation of the cerebral circulation: bedside assessment and clinical implications. Crit Care. 2016; 20:129.12. Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009; 10:373–86.13. Selb J, Wu KC, Sutin J, Lin PI, Farzam P, Bechek S, et al. Prolonged monitoring of cerebral blood flow and autoregulation with diffuse correlation spectroscopy in neurocritical care patients. Neurophotonics. 2018; 5:045005.14. Donnelly J, Aries MJ, Czosnyka M. Further understanding of cerebral autoregulation at the bedside: possible implications for future therapy. Expert Rev Neurother. 2015; 15:169–85.15. Crippa IA, Subirà C, Vincent JL, Fernandez RF, Hernandez SC, Cavicchi FZ, et al. Impaired cerebral autoregulation is associated with brain dysfunction in patients with sepsis. Crit Care. 2018; 22:327.16. Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med. 2006; 34:1783–8.17. Klein SP, Depreitere B, Meyfroidt G. How I monitor cerebral autoregulation. Crit Care. 2019; 23:160.18. Li C, Wu PM, Wu Z, Limnuson K, Mehan N, Mozayan C, et al. Highly accurate thermal flow microsensor for continuous and quantitative measurement of cerebral blood flow. Biomed Microdevices. 2015; 17:87.19. Li C, Wu PM, Hartings JA, Wu Z, Ahn CH, LeDoux D, et al. Smart catheter flow sensor for real-time continuous regional cerebral blood flow monitoring. Appl Phys Lett. 2011; 99:233705.20. Mintun MA, Lundstrom BN, Snyder AZ, Vlassenko AG, Shulman GL, Raichle ME. Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci U S A. 2001; 98:6859–64.21. Wolf S, Vajkoczy P, Dengler J, Schürer L, Horn P. Drift of the Bowman Hemedex® cerebral blood flow monitor between calibration cycles. Acta Neurochir Suppl. 2012; 114:187–90.22. Wilson MH, Wise D, Davies G, Lockey D. Emergency burr holes: “how to do it”. Scand J Trauma Resusc Emerg Med. 2012; 20:24.23. Huang CY, Lee PH, Lin SH, Chuang MT, Sun YT, Hung YC, et al. Remote cerebellar hemorrhage following supratentorial craniotomy. Neurol Res. 2012; 34:422–9.24. Bouzat P, Sala N, Payen JF, Oddo M. Beyond intracranial pressure: optimization of cerebral blood flow, oxygen, and substrate delivery after traumatic brain injury. Ann Intensive Care. 2013; 3:23.25. Depreitere B, Güiza F, Van den Berghe G, Schuhmann MU, Maier G, Piper I, et al. Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. J Neurosurg. 2014; 120:1451–7.26. Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin. 2016; 34:465–77.27. Donnelly J, Czosnyka M, Adams H, Robba C, Steiner LA, Cardim D, et al. Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med. 2017; 45:1464–71.28. Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley RB, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010; 41:1951–6.29. Tsalach A, Ratner E, Lokshin S, Silman Z, Breskin I, Budin N, et al. Cerebral autoregulation real-time monitoring. PLoS One. 2016; 11:e0161907.30. Wang X, Yang B, Ma Y, Gao P, Wang Y, Chen Y, et al. Comparison of monitoring of cerebral blood flow by c-FLOW and transcranial Doppler in carotid endarterectomy. World Neurosurg. 2018; 111:e686–92.31. Siegler JE, Martin-Schild S. Early neurological deterioration (END) after stroke: the END depends on the definition. Int J Stroke. 2011; 6:211–2.32. Lord AS, Gilmore E, Choi HA, Mayer SA; VISTA-ICH Collaboration. Time course and predictors of neurological deterioration after intracerebral hemorrhage. Stroke. 2015; 46:647–52.33. Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997; 41:11–9.34. Sviri GE, Aaslid R, Douville CM, Moore A, Newell DW. Time course for autoregulation recovery following severe traumatic brain injury. J Neurosurg. 2009; 111:695–700.35. Rickards CA, Tzeng YC. Arterial pressure and cerebral blood flow variability: friend or foe?: a review. Front Physiol. 2014; 5:120.36. Jaggi JL, Obrist WD, Gennarelli TA, Langfitt TW. Relationship of early cerebral blood flow and metabolism to outcome in acute head injury. J Neurosurg. 1990; 72:176–82.37. Clifton GL, Miller ER, Choi SC, Levin HS. Fluid thresholds and outcome from severe brain injury. Crit Care Med. 2002; 30:739–45.38. Philip S, Chaiwat O, Udomphorn Y, Moore A, Zimmerman JJ, Armstead W, et al. Variation in cerebral blood flow velocity with cerebral perfusion pressure >40 mm Hg in 42 children with severe traumatic brain injury. Crit Care Med. 2009; 37:2973–8.39. Coles JP, Fryer TD, Smielewski P, Chatfield DA, Steiner LA, Johnston AJ, et al. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab. 2004; 24:202–11.40. Lee SC, Chen JF, Lee ST. Continuous regional cerebral blood flow monitoring in the neurosurgical intensive care unit. J Clin Neurosci. 2005; 12:520–3.41. Pedro T, Pereira P, Costa AS, Almeida F, Loureiro ML, Alfaiate T, et al. Systolic blood pressure variability within 120 hours of admission predicts the functional outcomes at discharge of patients with acute ischemic stroke. J Neurocrit Care. 2022; 15:32–8.42. Esmaeeli S, Hrdlicka CM, Bastos AB, Wang J, Gomez-Paz S, Hanafy KA, et al. Robotically assisted transcranial Doppler with artificial intelligence for assessment of cerebral vasospasm after subarachnoid hemorrhage. J Neurocrit Care. 2020; 13:32–40.43. Linsler S, Schmidtke M, Steudel WI, Kiefer M, Oertel J. Automated intracranial pressure-controlled cerebrospinal fluid external drainage with LiquoGuard. Acta Neurochir (Wien). 2013; 155:1589–95.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Continuous heart rate variability and electroencephalography monitoring in severe acute brain injury: a preliminary study
- Multimodality Monitoring in the Neurointensive Care Unit: A Special Perspective for Patients with Stroke
- Cerebral perfusion pressure optimization for the regulation of brain edema and intracranial pressure
- Association of 3 Stigmas of Cerebral Microangiopathy With Early Neurological Deterioration in Lacunar Infarction
- Diagnostic Criteria for Determination of Brain Death Using the Transcranial Doppler(TCD)