Acute Crit Care.
2021 May;36(2):151-161. 10.4266/acc.2020.00703.
Continuous heart rate variability and electroencephalography monitoring in severe acute brain injury: a preliminary study
- Affiliations
-
- 1Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Neurology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2516263
- DOI: http://doi.org/10.4266/acc.2020.00703
Abstract
- Background
Decreases in heart rate variability have been shown to be associated with poor outcomes in severe acute brain injury. However, it is unknown whether the changes in heart rate variability precede neurological deterioration in such patients. We explored the changes in heart rate variability measured by electrocardiography in patients who had neurological deterioration following severe acute brain injury, and examined the relationship between heart rate variability and electroencephalography parameters.
Methods
Retrospective analysis of 25 patients who manifested neurological deterioration after severe acute brain injury and underwent simultaneous electroencephalography plus electrocardiography monitoring.
Results
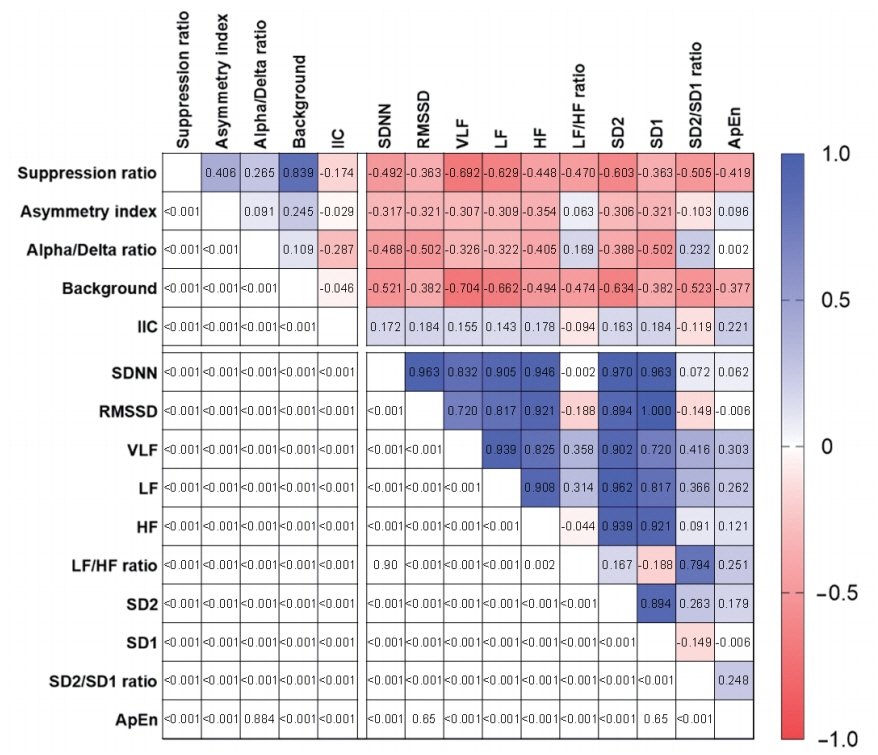
Eighteen electroencephalography channels and one simultaneously recorded electrocardiography channel were segmented into epochs of 120-second duration and processed to compute 10 heart rate variability parameters and three quantitative electroencephalography parameters. Raw electroencephalography of the epochs was also assessed by standardized visual interpretation and categorized based on their background abnormalities and ictalinterictal continuum patterns. The heart rate variability and electroencephalography parameters showed consistent changes in the 2-day period before neurological deterioration commenced. Remarkably, the suppression ratio and background abnormality of the electroencephalography parameters had significant reverse correlations with all heart rate variability parameters.
Conclusions
We observed a significantly progressive decline in heart rate variability from the day before the neurological deterioration events in patients with severe acute brain injury were first observed.
Figure
Reference
-
1. Geurts M, Macleod MR, van Thiel GJ, van Gijn J, Kappelle LJ, van der Worp HB. End-of-life decisions in patients with severe acute brain injury. Lancet Neurol. 2014; 13:515–24.
Article2. Stevens RD, Sutter R. Prognosis in severe brain injury. Crit Care Med. 2013; 41:1104–23.
Article3. Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy G, et al. The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a list of recommendations and additional conclusions: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care. 2014; 21 Suppl 2(Suppl 2):S282–96.4. Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994; 36:557–65.
Article5. Herman ST, Abend NS, Bleck TP, Chapman KE, Drislane FW, Emerson RG, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015; 32:87–95.6. Foreman B, Claassen J. Quantitative EEG for the detection of brain ischemia. In : Vincent JL, editor. Annual update in intensive care and emergency medicine 2012. Berlin, Heidelberg: Springer Berlin Heidelberg;2012. p. 746–58.7. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996; 17:354–81.8. de Castilho FM, Ribeiro ALP, Nobre V, Barros G, de Sousa MR. Heart rate variability as predictor of mortality in sepsis: a systematic review. PLoS One. 2018; 13:e0203487.
Article9. Karmali SN, Sciusco A, May SM, Ackland GL. Heart rate variability in critical care medicine: a systematic review. Intensive Care Med Exp. 2017; 5:33.
Article10. Admiraal MM, Gilmore EJ, Van Putten MJ, Zaveri HP, Hirsch LJ, Gaspard N. Disruption of brain-heart coupling in sepsis. J Clin Neurophysiol. 2017; 34:413–20.
Article11. Cooke WH, Salinas J, Convertino VA, Ludwig DA, Hinds D, Duke JH, et al. Heart rate variability and its association with mortality in prehospital trauma patients. J Trauma. 2006; 60:363–70.
Article12. Grogan EL, Morris JA Jr, Norris PR, France DJ, Ozdas A, Stiles RA, et al. Reduced heart rate volatility: an early predictor of death in trauma patients. Ann Surg. 2004; 240:547–54.13. Hendén PL, Söndergaard S, Rydenhag B, Reinsfelt B, Ricksten SE, Aneman A. Can baroreflex sensitivity and heart rate variability predict late neurological outcome in patients with traumatic brain injury? J Neurosurg Anesthesiol. 2014; 26:50–9.
Article14. Rapenne T, Moreau D, Lenfant F, Vernet M, Boggio V, Cottin Y, Freysz M. Could heart rate variability predict outcome in patients with severe head injury? A pilot study. J Neurosurg Anesthesiol. 2001; 13:260–8.15. Baillard C, Vivien B, Mansier P, Mangin L, Jasson S, Riou B, et al. Brain death assessment using instant spectral analysis of heart rate variability. Crit Care Med. 2002; 30:306–10.
Article16. Conci F, Di Rienzo M, Castiglioni P. Blood pressure and heart rate variability and baroreflex sensitivity before and after brain death. J Neurol Neurosurg Psychiatry. 2001; 71:621–31.
Article17. Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013; 30:1–27.18. Lee H, Mizrahi MA, Hartings JA, Sharma S, Pahren L, Ngwenya LB, et al. Continuous electroencephalography after moderate to severe traumatic brain injury. Crit Care Med. 2019; 47:574–582.
Article19. Rodriguez Ruiz A, Vlachy J, Lee JW, Gilmore EJ, Ayer T, Haider HA, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically Ill patients. JAMA Neurol. 2017; 74:181–8.
Article20. Megjhani M, Kaffashi F, Terilli K, Alkhachroum A, Esmaeili B, Doyle KW, et al. Heart rate variability as a biomarker of neurocardiogenic injury after subarachnoid hemorrhage. Neurocrit Care. 2020; 32:162–71.
Article21. Goulding RM, Stevenson NJ, Murray DM, Livingstone V, Filan PM, Boylan GB. Heart rate variability in hypoxic ischemic encephalopathy: correlation with EEG grade and 2-y neurodevelopmental outcome. Pediatr Res. 2015; 77:681–7.
Article22. Vergales BD, Zanelli SA, Matsumoto JA, Goodkin HP, Lake DE, Moorman JR, et al. Depressed heart rate variability is associated with abnormal EEG, MRI, and death in neonates with hypoxic ischemic encephalopathy. Am J Perinatol. 2014; 31:855–62.
Article23. Beniczky S, Hirsch LJ, Kaplan PW, Pressler R, Bauer G, Aurlien H, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013; 54 Suppl 6:28–9.
Article24. Rosenthal ES, Biswal S, Zafar SF, O’Connor KL, Bechek S, Shenoy AV, et al. Continuous electroencephalography predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective study of diagnostic accuracy. Ann Neurol. 2018; 83:958–69.
Article25. Claassen J, Hirsch LJ, Kreiter KT, Du EY, Connolly ES, Emerson RG, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004; 115:2699–710.
Article26. Gollwitzer S, Groemer T, Rampp S, Hagge M, Olmes D, Huttner HB, et al. Early prediction of delayed cerebral ischemia in subarachnoid hemorrhage based on quantitative EEG: a prospective study in adults. Clin Neurophysiol. 2015; 126:1514–23.
Article27. Foreman B, Albers D, Schmidt JM, Falo CM, Velasquez A, Connolly ES, et al. Intracortical electrophysiological correlates of blood flow after severe SAH: a multimodality monitoring study. J Cereb Blood Flow Metab. 2018; 38:506–17.
Article28. Carrasco S, Gaitán MJ, González R, Yánez O. Correlation among Poincaré plot indexes and time and frequency domain measures of heart rate variability. J Med Eng Technol. 2001; 25:240–8.29. Counihan PJ, Fei L, Bashir Y, Farrell TG, Haywood GA, McKenna WJ. Assessment of heart rate variability in hypertrophic cardiomyopathy: association with clinical and prognostic features. Circulation. 1993; 88(4 Pt 1):1682–90.
Article30. Fontolliet T, Gianella P, Pichot V, Barthélémy JC, Gasche-Soccal P, Ferretti G, et al. Heart rate variability and baroreflex sensitivity in bilateral lung transplant recipients. Clin Physiol Funct Imaging. 2018; 38:872–80.
Article31. Tibby SM, Frndova H, Durward A, Cox PN. Novel method to quantify loss of heart rate variability in pediatric multiple organ failure. Crit Care Med. 2003; 31:2059–67.
Article32. Swor DE, Thomas LF, Maas MB, Grimaldi D, Manno EM, Sorond FA, et al. Admission heart rate variability is associated with fever development in patients with intracerebral hemorrhage. Neurocrit Care. 2019; 30:244–50.
Article33. Sykora M, Czosnyka M, Liu X, Donnelly J, Nasr N, Diedler J, et al. Autonomic impairment in severe traumatic brain injury: a multimodal neuromonitoring study. Crit Care Med. 2016; 44:1173–81.34. Mowery NT, Norris PR, Riordan W, Jenkins JM, Williams AE, Morris JA Jr. Cardiac uncoupling and heart rate variability are associated with intracranial hypertension and mortality: a study of 145 trauma patients with continuous monitoring. J Trauma. 2008; 65:621–7.
Article35. Biswas AK, Scott WA, Sommerauer JF, Luckett PM. Heart rate variability after acute traumatic brain injury in children. Crit Care Med. 2000; 28:3907–12.
Article36. Naver HK, Blomstrand C, Wallin BG. Reduced heart rate variability after right-sided stroke. Stroke. 1996; 27:247–51.
Article37. Wang YM, Wu HT, Huang EY, Kou YR, Hseu SS. Heart rate variability is associated with survival in patients with brain metastasis: a preliminary report. Biomed Res Int. 2013; 2013:503421.
Article38. Papaioannou V, Giannakou M, Maglaveras N, Sofianos E, Giala M. Investigation of heart rate and blood pressure variability, baroreflex sensitivity, and approximate entropy in acute brain injury patients. J Crit Care. 2008; 23:380–6.
Article39. Haji-Michael PG, Vincent JL, Degaute JP, van de Borne P. Power spectral analysis of cardiovascular variability in critically ill neurosurgical patients. Crit Care Med. 2000; 28:2578–83.
Article40. Piantino JA, Lin A, Crowder D, Williams CN, Perez-Alday E, Tereshchenko LG, et al. Early heart rate variability and electroencephalographic abnormalities in acutely brain-injured children who progress to brain death. Pediatr Crit Care Med. 2019; 20:38–46.
Article41. Machado C, Estevez M, Perez-Nellar J, Schiavi A. Residual vasomotor activity assessed by heart rate variability in a braindead case. BMJ Case Rep. 2015; 2015:bcr2014205677.
Article42. Jurak P, Halamek J, Vondra V, Kruzliak P, Sramek V, Cundrle I, et al. Respiratory induced heart rate variability during slow mechanical ventilation: marker to exclude brain death patients. Wien Klin Wochenschr. 2017; 129:251–8.43. Witsch J, Frey HP, Schmidt JM, Velazquez A, Falo CM, Reznik M, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol. 2017; 74:301–9.
Article44. Struck AF, Westover MB, Hall LT, Deck GM, Cole AJ, Rosenthal ES. Metabolic correlates of the ictal-interictal continuum: FDG-PET during continuous EEG. Neurocrit Care. 2016; 24:324–31.
Article45. Vespa P, Tubi M, Claassen J, Buitrago-Blanco M, McArthur D, Velazquez AG, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016; 79:579–90.
Article46. Moridani MK, Farhadi H. Heart rate variability as a biomarker for epilepsy seizure prediction. Bratisl Lek Listy. 2017; 118:3–8.
Article47. Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol. 2013; 4:26.
Article48. Guild SJ, Saxena UA, McBryde FD, Malpas SC, Ramchandra R. Intracranial pressure influences the level of sympathetic tone. Am J Physiol Regul Integr Comp Physiol. 2018; 315:R1049–53.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multimodality Monitoring in the Neurointensive Care Unit: A Special Perspective for Patients with Stroke
- Global Synchronization Index as an Indicator for Tracking Cognitive Function Changes in a Traumatic Brain Injury Patient: A Case Report
- Heart and Brain Interaction of Psychiatric Illness: A Review Focused on Heart Rate Variability, Cognitive Function, and Quantitative Electroencephalography
- Assessment of Autonomic Function in Post-Acute Ambulatory Patients with Mild or Moderate Traumatic Brain Injury Using the Analysis of Heart Rate Variability
- The Relationship and Mechanism Underlying the Effect of Conscious Breathing on the Autonomic Nervous System and Brain Waves