Endocrinol Metab.
2024 Jun;39(3):489-499. 10.3803/EnM.2023.1888.
Reference Standards for C-Peptide in Korean Population: A Korean Endocrine Hormone Reference Standard Data Center Study
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
- 2Department of Internal Medicine, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University Chuncheon Sacred Heart Hospital, College of Medicine, Hallym University, Chuncheon, Korea
- 4Department of Internal Medicine, Nowon Eulji Medical Center, Eulji University School of Medicine, Seoul, Korea
- 5Korea Research Institute of Standards and Science, Daejeon, Korea
- 6Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
- 7Research Institute of Metabolism and Inflammation, Yonsei University Wonju College of Medicine, Wonju, Korea
- KMID: 2556641
- DOI: http://doi.org/10.3803/EnM.2023.1888
Abstract
- Background
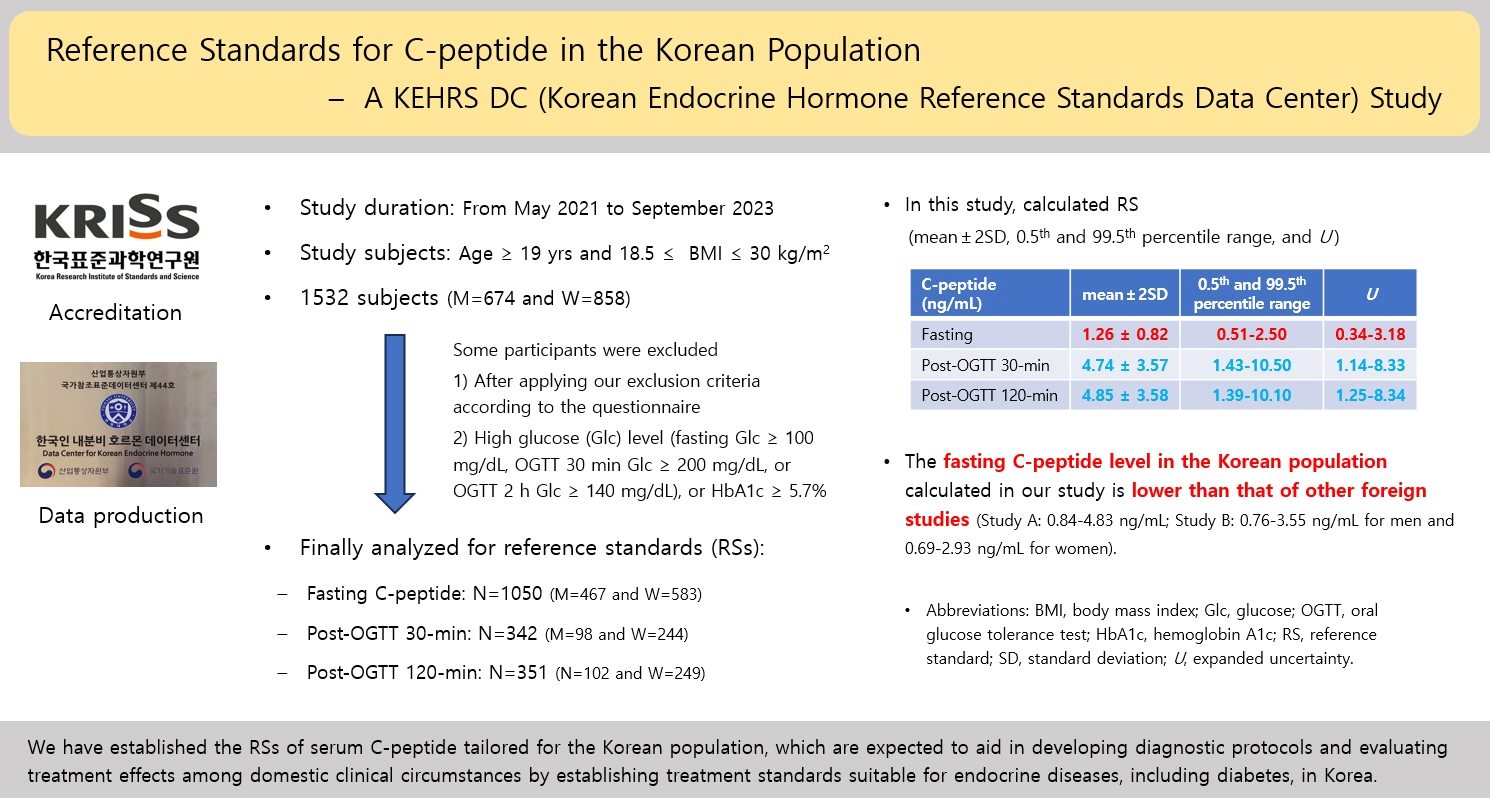
The Korean Endocrine Hormone Reference Standard Data Center (KEHRS DC) has created reference standards (RSs) for endocrine hormones since 2020. This study is the first of its kind, wherein the KEHRS DC established RSs for serum Cpeptide levels in a healthy Korean population.
Methods
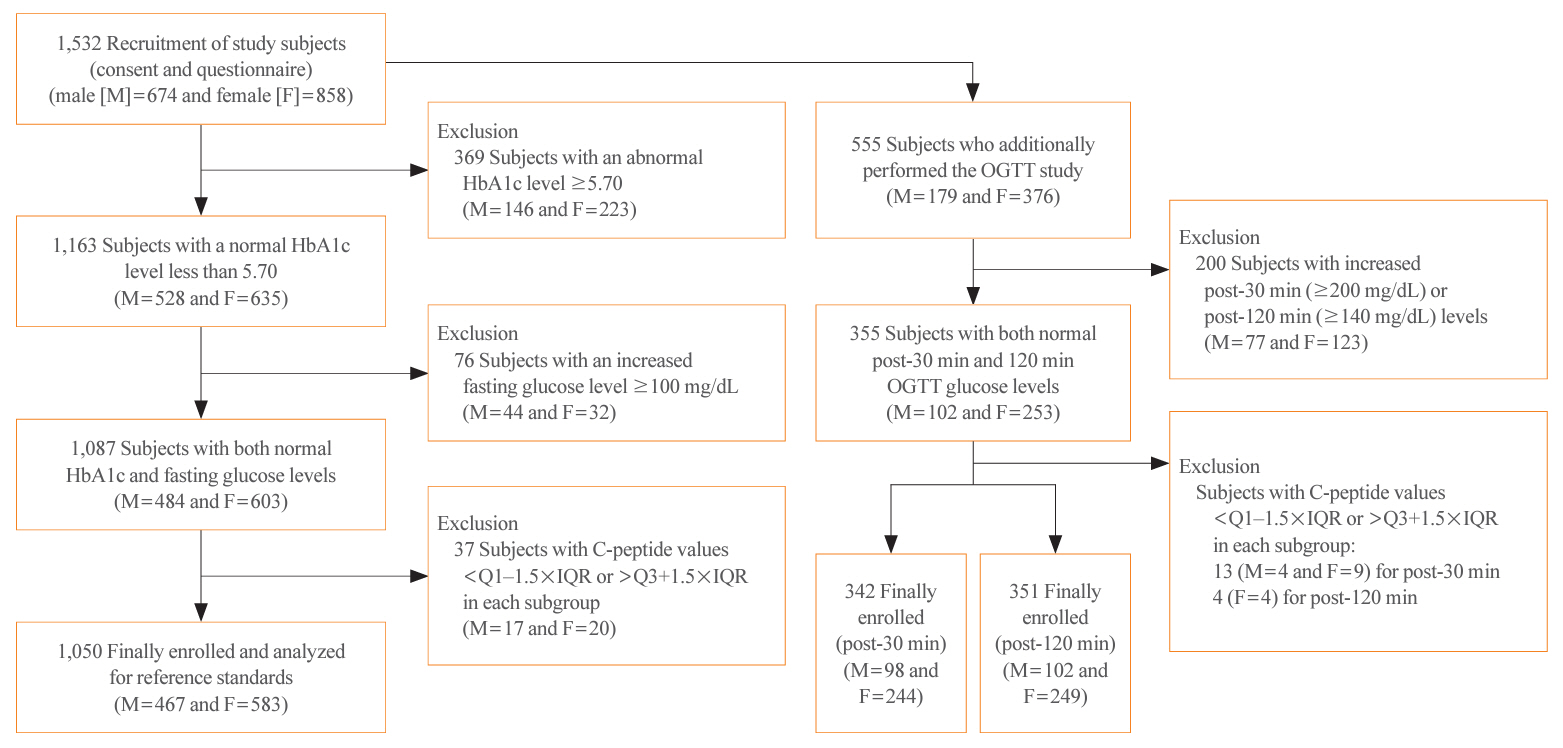
Healthy Korean adults were recruited from May 2021 to September 2023. After excluding participants according to our criteria, serum samples were collected; each participant could then choose between fasting glucose only or fasting glucose plus an oral glucose tolerance test (OGTT). If their sample showed high glucose (≥100 mg/dL) or hemoglobin A1c (HbA1c) (≥5.70%), their C-peptide levels were excluded from analyzing the RSs.
Results
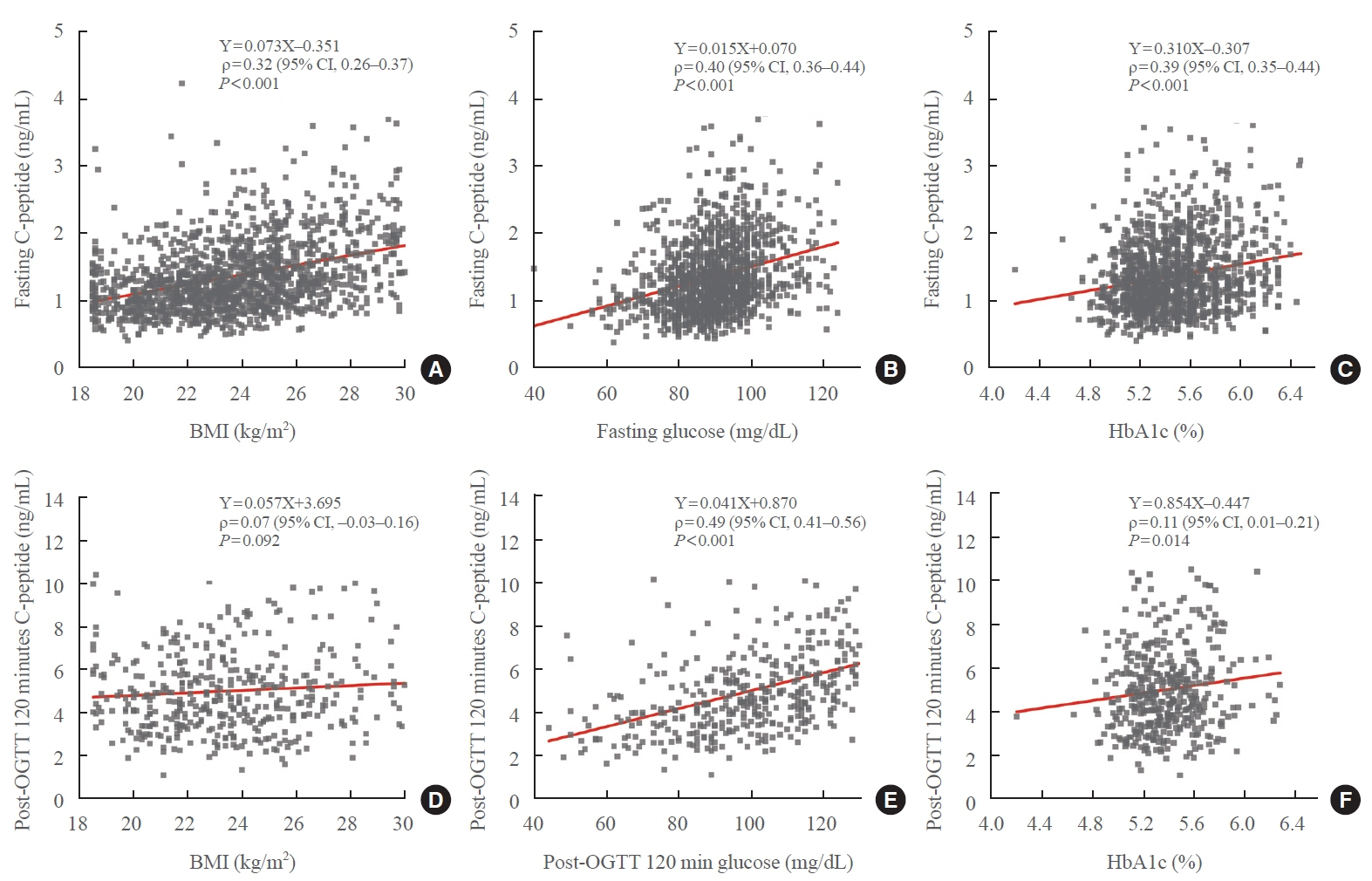
A total of 1,532 participants were recruited; however, only the data of 1,050 participants were analyzed after excluding those whose samples showed hyperglycemia or high HbA1c. Post-30-minute OGTT data from 342 subjects and post-120-minute OGTT data from 351 subjects were used. The means±2 standard deviations and expanded uncertainties of fasting, post-30-minute and 120-minute OGTT C-peptide levels were 1.26±0.82 and 0.34–3.18, 4.74±3.57 and 1.14–8.33, and 4.85±3.58 and 1.25–8.34 ng/mL, respectively. Serum C-peptide levels correlated with obesity, serum glucose levels, and HbA1c levels.
Conclusion
The RSs for serum C-peptide levels established in this study are expected to be useful in both clinical and related fields.
Keyword
Figure
Reference
-
1. Kelly J, Raizman JE, Bevilacqua V, Chan MK, Chen Y, Quinn F, et al. Complex reference value distributions and partitioned reference intervals across the pediatric age range for 14 specialized biochemical markers in the CALIPER cohort of healthy community children and adolescents. Clin Chim Acta. 2015; 450:196–202.
Article2. Farrell CL, Nguyen L, Carter AC. Parathyroid hormone: data mining for age-related reference intervals in adults. Clin Endocrinol (Oxf). 2018; 88:311–7.
Article3. Chung JH. Update on thyroid hormone levels and thyroid dysfunction in the Korean population based on data from the Korea National Health and Nutrition Examination Survey VI (2013 to 2015). Endocrinol Metab (Seoul). 2020; 35:7–13.
Article4. Choi R, Lee SG, Lee EH. Reference intervals of anti-Müllerian hormone in Korean women. J Clin Lab Anal. 2022; 36:e24525.
Article5. Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009; 94:907–13.
Article6. Kuzmenko NV, Tsyrlin VA, Pliss MG, Galagudza MM. Seasonal variations in levels of human thyroid-stimulating hormone and thyroid hormones: a meta-analysis. Chronobiol Int. 2021; 38:301–17.
Article7. Kaaja RJ, Leinonen A, Moore P, Yandle T, Frampton CM, Nicholls MG. Effect of changes in body posture on vasoactive hormones in pre-eclamptic women. J Hum Hypertens. 2004; 18:789–94.
Article8. Higgins V, Chan MK, Nieuwesteeg M, Hoffman BR, Bromberg IL, Gornall D, et al. Transference of CALIPER pediatric reference intervals to biochemical assays on the Roche cobas 6000 and the Roche Modular P. Clin Biochem. 2016; 49:139–49.
Article9. Ohn JH, Kwak SH, Cho YM, Lim S, Jang HC, Park KS, et al. 10-Year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: a community-based prospective cohort study. Lancet Diabetes Endocrinol. 2016; 4:27–34.
Article10. Ahlqvist E, Prasad RB, Groop L. Subtypes of type 2 diabetes determined from clinical parameters. Diabetes. 2020; 69:2086–93.
Article11. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011; 34(Suppl 1):S62–9.12. Little RR, Rohlfing CL, Tennill AL, Madsen RW, Polonsky KS, Myers GL, et al. Standardization of C-peptide measurements. Clin Chem. 2008; 54:1023–6.
Article13. Clinical Laboratory Standards Institute. CLSI document EP29-A. Expression of measurement uncertainty in laboratory medicine: approved guideline. Wayne: CLSI;2012.14. Kim JD, Kang SJ, Lee MK, Park SE, Rhee EJ, Park CY, et al. C-peptide-based index is more related to incident type 2 diabetes in non-diabetic subjects than insulin-based index. Endocrinol Metab (Seoul). 2016; 31:320–7.15. World Health Organization; WHO Expert Committee on Biological Standardization. WHO International Collaborative Study of the proposed 1st IS for human C-peptide: Expert Committee on Biological Standardization. Geneva: WHO;2015.16. Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013; 30:803–17.17. Maddaloni E, Bolli GB, Frier BM, Little RR, Leslie RD, Pozzilli P, et al. C-peptide determination in the diagnosis of type of diabetes and its management: a clinical perspective. Diabetes Obes Metab. 2022; 24:1912–26.18. Shim WS, Kim SK, Kim HJ, Park SE, Kang ES, Rhee YM, et al. Clinical meaning of postprandial insulin secretory function in Korean type 2 diabetes mellitus. J Korean Diabetes Assoc. 2005; 29:367–77.19. Chen J, Huang Y, Liu C, Chi J, Wang Y, Xu L. The role of C-peptide in diabetes and its complications: an updated review. Front Endocrinol (Lausanne). 2023; 14:1256093.
Article20. Luppi P, Geng X, Cifarelli V, Drain P, Trucco M. C-peptide is internalized in human endothelial and vascular smooth muscle cells via early endosomes. Diabetologia. 2009; 52:2218–28.
Article21. Kim JD, Lee WY. Insulin secretory capacity and insulin resistance in Korean type 2 diabetes mellitus patients. Endocrinol Metab (Seoul). 2016; 31:354–60.
Article22. Katz LE, Jawad AF, Ganesh J, Abraham M, Murphy K, Lipman TH. Fasting C-peptide and insulin-like growth factor-binding protein-1 levels help to distinguish childhood type 1 and type 2 diabetes at diagnosis. Pediatr Diabetes. 2007; 8:53–9.
Article23. Berger B, Stenstrom G, Sundkvist G. Random C-peptide in the classification of diabetes. Scand J Clin Lab Invest. 2000; 60:687–93.
Article24. Torn C, Landin‐Olsson M, Schersten B. Predictability of C‐peptide for autoimmune diabetes in young adult diabetic patients. Pract Diabetes Int. 2001; 18:83–8.
Article25. Larsen PB, Linneberg A, Hansen T, Friis-Hansen L. Reference intervals for C-peptide and insulin derived from a general adult Danish population. Clin Biochem. 2017; 50:408–13.
Article26. Borai A, Ichihara K, Masaud A, Tamimi W, Bahijri S, Armbuster D, et al. Establishment of reference intervals for immunoassay analytes of adult population in Saudi Arabia. Clin Chem Lab Med. 2020; 58:1302–13.
Article27. Oh J, Kim JH, Park HD. Clinical utility and cross-reactivity of insulin and C-peptide assays by the Lumipulse G1200 system. Ann Lab Med. 2018; 38:530–7.
Article28. Leighton E, Sainsbury CA, Jones GC. A practical review of C-peptide testing in diabetes. Diabetes Ther. 2017; 8:475–87.
Article29. Chon S, Rhee SY, Oh S, Kim SW, Kim JW, Kim YS, et al. Pancreas beta-cell function impaired in obese normal young adults. Korean J Obes. 2006; 15:146–58.30. Clinical Laboratory Standards Institute. CLSI document EP28-A3c. Defining, establishing, and verifying reference intervals in the clinical laboratory: approved guideline. 3rd ed. Wayne: CLSI;2010.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of C-peptide Test on Modular E170 Electrochemiluminescent Autoanalyzer
- Evaluation of Growth between Breast-Fed and Formula-Fed Korean Infants from 1 to 3 Postpartum Months: Compared with the Korean Standard and NCHS Reference
- Ulnar Nerve Conduction Studies: Reference Standards with Extended Uncertainty in Healthy South Korean Adults
- Collaborative Study to Establish National Reference Standards for Anti-HIV-1 Antibody
- Development of nutrient-based nutritional standards for foodservice at shelters during disasters in the Republic of Korea