Endocrinol Metab.
2024 Jun;39(3):468-478. 10.3803/EnM.2024.1923.
Utilizing Immunoglobulin G4 Immunohistochemistry for Risk Stratification in Patients with Papillary Thyroid Carcinoma Associated with Hashimoto Thyroiditis
- Affiliations
-
- 1Department of Biomedicine and Health Sciences, The Catholic University of Korea, Seoul, Korea
- 2Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Diagnostic Pathology and Cytology, Kuma Hospital, Kobe, Japan
- KMID: 2556639
- DOI: http://doi.org/10.3803/EnM.2024.1923
Abstract
- Background
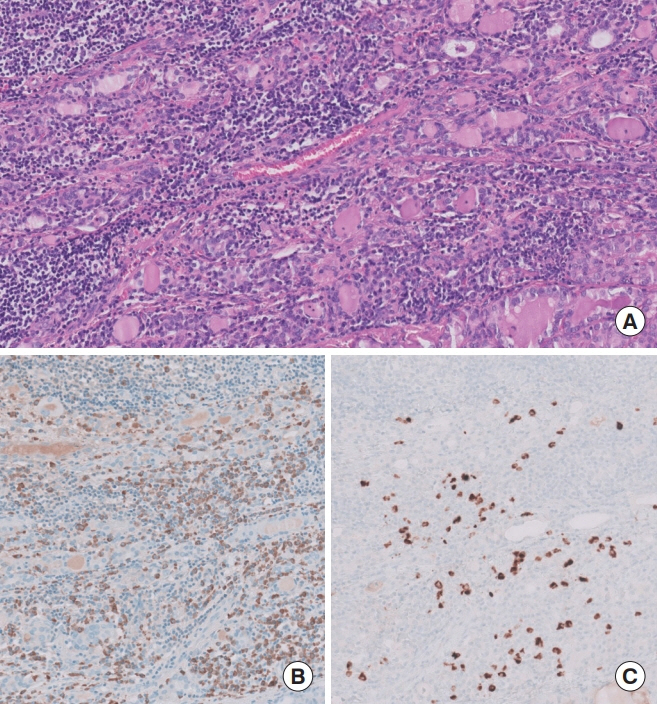
Hashimoto thyroiditis (HT) is suspected to correlate with papillary thyroid carcinoma (PTC) development. While some HT cases exhibit histologic features of immunoglobulin G4 (IgG4)-related disease, the relationship of HT with PTC progression remains unestablished.
Methods
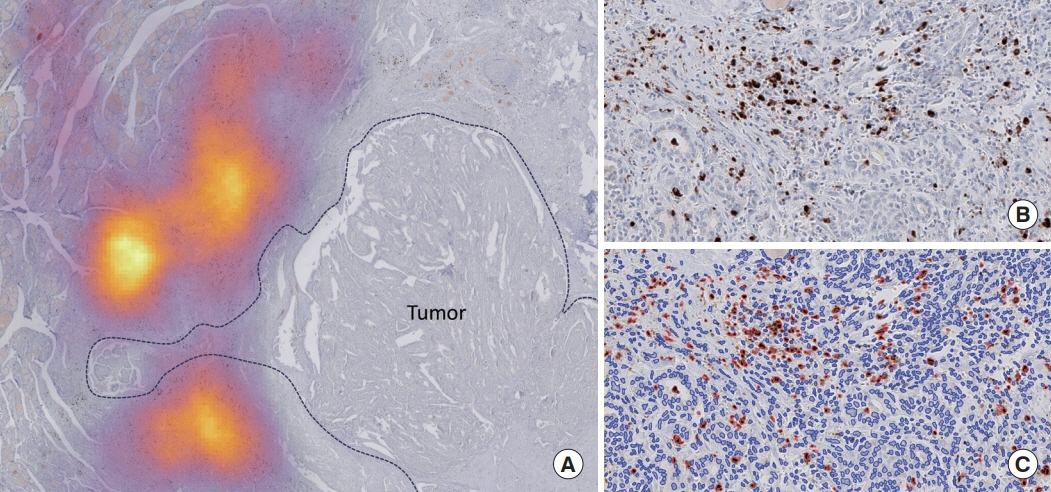
This cross-sectional study included 426 adult patients with PTC (≥1 cm) undergoing thyroidectomy at an academic thyroid center. HT was identified based on its typical histologic features. IgG4 and IgG immunohistochemistry were performed. Wholeslide images of immunostained slides were digitalized. Positive plasma cells per 2 mm2 were counted using QuPath and a pre-trained deep learning model. The primary outcome was tumor structural recurrence post-surgery.
Results
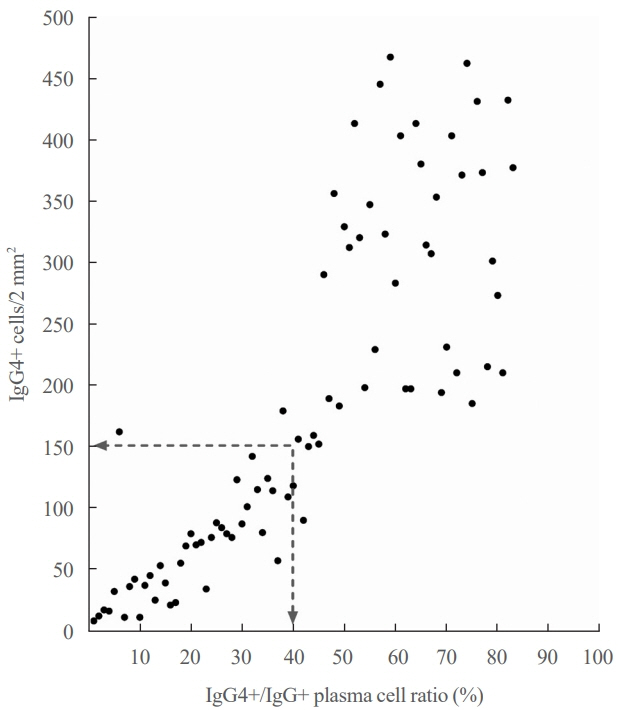
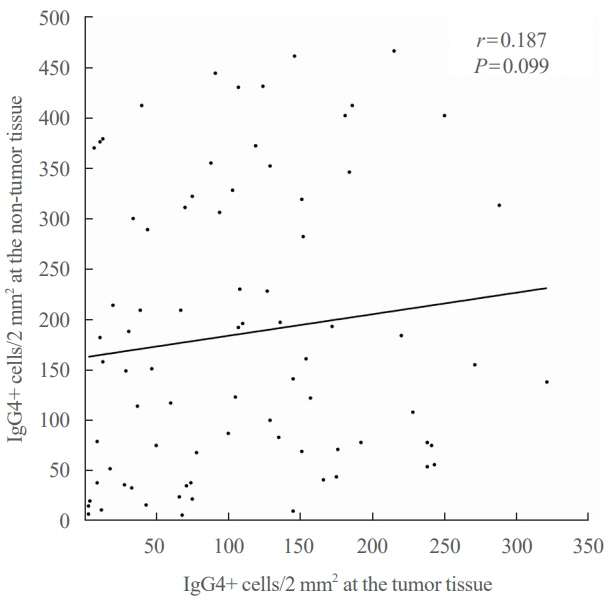
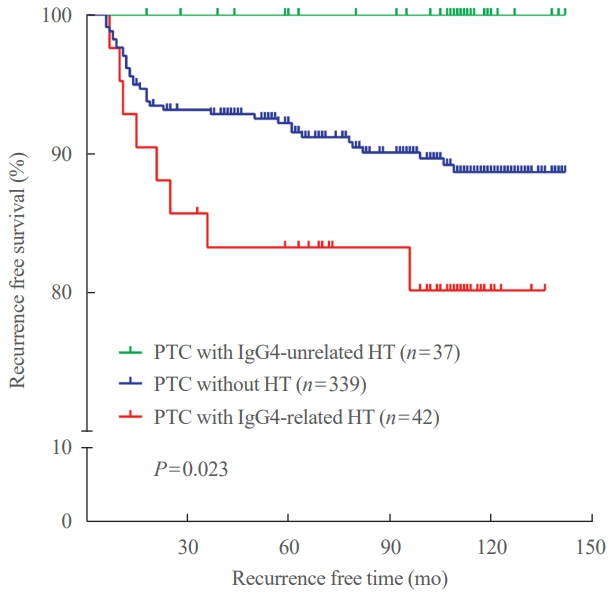
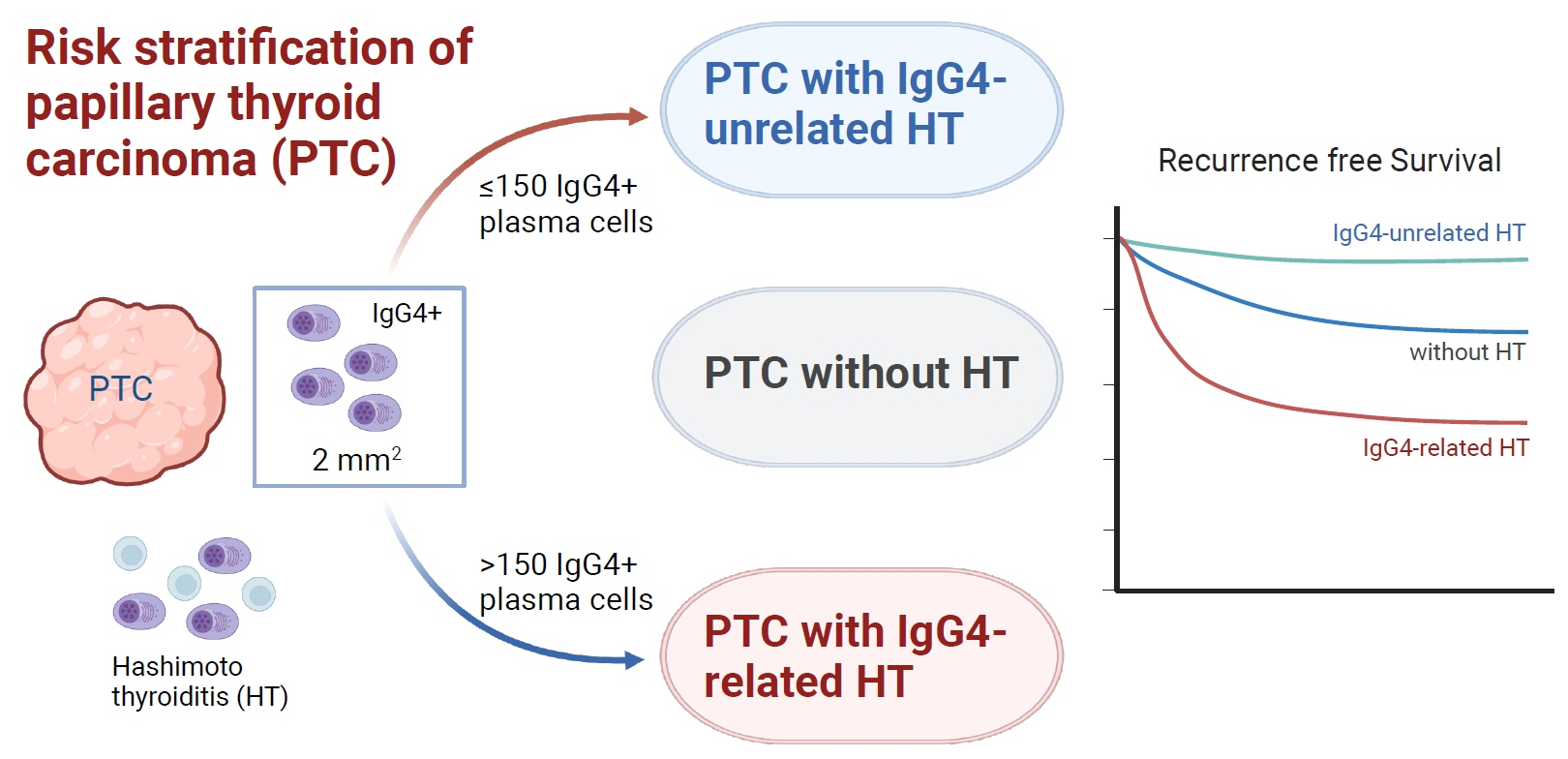
Among the 426 PTC patients, 79 were diagnosed with HT. With a 40% IgG4 positive/IgG plasma cell ratio as the threshold for diagnosing IgG4-related disease, a cutoff value of >150 IgG4 positive plasma cells per 2 mm2 was established. According to this criterion, 53% (43/79) of HT patients were classified as IgG4-related. The IgG4-related HT subgroup presented a more advanced cancer stage than the IgG4-non-related HT group (P=0.038). The median observation period was 109 months (range, 6 to 142). Initial assessment revealed 43 recurrence cases. Recurrence-free survival periods showed significant (P=0.023) differences, with patients with IgG4 non-related HT showing the longest period, followed by patients without HT and those with IgG4-related HT.
Conclusion
This study effectively stratified recurrence risk in PTC patients based on HT status and IgG4-related subtypes. These findings may contribute to better-informed treatment decisions and patient care strategies.
Keyword
Figure
Reference
-
1. Ragusa F, Fallahi P, Elia G, Gonnella D, Paparo SR, Giusti C, et al. Hashimotos’ thyroiditis: epidemiology, pathogenesis, clinic and therapy. Best Pract Res Clin Endocrinol Metab. 2019; 33:101367.
Article2. Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014; 13:391–7.
Article3. Lorini R, Gastaldi R, Traggiai C, Perucchin PP. Hashimoto’s thyroiditis. Pediatr Endocrinol Rev. 2003; 1 Suppl 2:205–11.4. Gatto F, Barbieri F, Gatti M, Wurth R, Schulz S, Ravetti JL, et al. Balance between somatostatin and D2 receptor expression drives TSH-secreting adenoma response to somatostatin analogues and dopastatins. Clin Endocrinol (Oxf). 2012; 76:407–14.
Article5. Moon S, Chung HS, Yu JM, Yoo HJ, Park JH, Kim DS, et al. Associations between Hashimoto thyroiditis and clinical outcomes of papillary thyroid cancer: a meta-analysis of observational studies. Endocrinol Metab (Seoul). 2018; 33:473–84.
Article6. Hussein O, Abdelwahab K, Hamdy O, Awny S, Megahed NA, Hafez MT, et al. Thyroid cancer associated with Hashimoto thyroiditis: similarities and differences in an endemic area. J Egypt Natl Canc Inst. 2020; 32:7.
Article7. Osborne D, Choudhary R, Vyas A, Kampa P, Abbas LF, Chigurupati HD, et al. Hashimoto’s thyroiditis effects on papillary thyroid carcinoma outcomes: a systematic review. Cureus. 2022; 14:e28054.
Article8. Xu S, Huang H, Qian J, Liu Y, Huang Y, Wang X, et al. Prevalence of Hashimoto thyroiditis in adults with papillary thyroid cancer and its association with cancer recurrence and outcomes. JAMA Netw Open. 2021; 4:e2118526.
Article9. Li Y, Inomata K, Nishihara E, Kakudo K. IgG4 thyroiditis in the Asian population. Gland Surg. 2020; 9:1838–46.
Article10. Li Y, Bai Y, Liu Z, Ozaki T, Taniguchi E, Mori I, et al. Immunohistochemistry of IgG4 can help subclassify Hashimoto’s autoimmune thyroiditis. Pathol Int. 2009; 59:636–41.
Article11. Han X, Zhang P, Li J, Liu Z, Lu H, Luo X, et al. Clinical features and treatment efficacy for IgG4-related thyroiditis. Orphanet J Rare Dis. 2021; 16:324.
Article12. Bledsoe JR, Della-Torre E, Rovati L, Deshpande V. IgG4- related disease: review of the histopathologic features, differential diagnosis, and therapeutic approach. APMIS. 2018; 126:459–76.
Article13. Adams SH, Gitto L, Serinelli S, Curtiss C. Review of IgG4-related Hashimoto thyroiditis with best practice recommendations for diagnosis and reporting. Adv Anat Pathol. 2022; 29:97–107.
Article14. Cree IA, Tan PH, Travis WD, Wesseling P, Yagi Y, White VA, et al. Counting mitoses: SI(ze) matters! Mod Pathol. 2021; 34:1651–7.
Article15. Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012; 25:1181–92.
Article16. Jeong YM, Cho H, Kim TM, Kim Y, Jeon S, Bychkov A, et al. CD73 overexpression promotes progression and recurrence of papillary thyroid carcinoma. Cancers (Basel). 2020; 12:3042.
Article17. Kim Y, Kim MH, Jeon S, Kim J, Kim C, Bae JS, et al. Prognostic implication of histological features associated with EHD2 expression in papillary thyroid carcinoma. PLoS One. 2017; 12:e0174737.
Article18. Bychkov A, Jung CK. Aberrant expression of CD20 in thyroid cancer and its clinicopathologic significance. Hum Pathol. 2018; 71:74–83.
Article19. Choden S, Keelawat S, Jung CK, Bychkov A. VE1 immunohistochemistry improves the limit of genotyping for detecting BRAFV600E mutation in papillary thyroid cancer. Cancers (Basel). 2020; 12:596.
Article20. Oh EJ, Bychkov A, Cho H, Kim TM, Bae JS, Lim DJ, et al. Prognostic implications of CD10 and CD15 expression in papillary thyroid carcinoma. Cancers (Basel). 2020; 12:1413.
Article21. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022; 33:27–63.
Article22. Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization classification of thyroid tumors: a standardized diagnostic approach. Endocrinol Metab (Seoul). 2022; 37:703–18.
Article23. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. New York: Springer;2017. 873–90.24. Bankhead P, Loughrey MB, Fernandez JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017; 7:16878.
Article25. Takeshima K, Li Y, Kakudo K, Hirokawa M, Nishihara E, Shimatsu A, et al. Proposal of diagnostic criteria for IgG4- related thyroid disease. Endocr J. 2021; 68:1–6.
Article26. Jeong HJ, Shin SJ, Lim BJ. Overview of IgG4-related tubulointerstitial nephritis and its mimickers. J Pathol Transl Med. 2016; 50:26–36.
Article27. Sim J, Koh HH, Choi S, Chu J, Kim TS, Kim H, et al. Pulmonary nodular lymphoid hyperplasia with mass-formation: clinicopathologic characteristics of nine cases and review of the literature. J Pathol Transl Med. 2018; 52:211–8.
Article28. Ramakrishna B, Yewale R, Vijayakumar K, Radhakrishna P, Ramakrishna BS. Gastric IgG4-related disease presenting as a mass lesion and masquerading as a gastrointestinal stromal tumor. J Pathol Transl Med. 2020; 54:258–62.
Article29. Kim MJ, Song TJ, Kim HJ, Kim SC, Kim MH, Hong SM. Coexisting mucinous cystic neoplasm of the pancreas and type 1 autoimmune pancreatitis. J Pathol Transl Med. 2019; 53:125–8.
Article30. Cree IA. From counting mitoses to Ki67 assessment: technical pitfalls in the new WHO Classification of Endocrine and Neuroendocrine Tumors. Endocr Pathol. 2022; 33:3–5.
Article31. Li Y, Wang X, Liu Z, Ma J, Lin X, Qin Y, et al. Hashimoto’s thyroiditis with increased IgG4-positive plasma cells: using thyroid-specific diagnostic criteria may identify early phase IgG4 thyroiditis. Thyroid. 2020; 30:251–61.
Article32. Li Y, Zhou G, Ozaki T, Nishihara E, Matsuzuka F, Bai Y, et al. Distinct histopathological features of Hashimoto’s thyroiditis with respect to IgG4-related disease. Mod Pathol. 2012; 25:1086–97.
Article33. Li Y, Nishihara E, Hirokawa M, Taniguchi E, Miyauchi A, Kakudo K. Distinct clinical, serological, and sonographic characteristics of hashimoto’s thyroiditis based with and without IgG4-positive plasma cells. J Clin Endocrinol Metab. 2010; 95:1309–17.
Article34. Yu Y, Yu N, Lu G, Li T, Zhang Y, Zhang J, et al. Hashimoto’s thyroiditis with elevated serum IgG4 concentrations is not equivalent to IgG4 Hashimoto’s thyroiditis. Clin Endocrinol (Oxf). 2018; 88:943–9.
Article35. Yu Y, Zhang J, Lu G, Li T, Zhang Y, Yu N, et al. Clinical relationship between IgG4-positive Hashimoto’s thyroiditis and papillary thyroid carcinoma. J Clin Endocrinol Metab. 2016; 101:1516–24.
Article36. Zhang J, Zhao L, Gao Y, Liu M, Li T, Huang Y, et al. A classification of Hashimoto’s thyroiditis based on immunohistochemistry for IgG4 and IgG. Thyroid. 2014; 24:364–70.
Article37. Deshpande V, Huck A, Ooi E, Stone JH, Faquin WC, Nielsen GP. Fibrosing variant of Hashimoto thyroiditis is an IgG4 related disease. J Clin Pathol. 2012; 65:725–8.
Article38. Raess PW, Habashi A, El Rassi E, Milas M, Sauer DA, Troxell ML. Overlapping morphologic and immunohistochemical features of Hashimoto thyroiditis and IgG4-related thyroid disease. Endocr Pathol. 2015; 26:170–7.
Article39. Lintusaari J, Vesaniemi E, Kalfert D, Ilvesaro J, Ludvikova M, Kholova I. IgG4-positive plasma cells in Hashimoto thyroiditis: IgG4-related disease or inflammation-related IgG4- positivity? APMIS. 2020; 128:531–8.
Article40. Jokisch F, Kleinlein I, Haller B, Seehaus T, Fuerst H, Kremer M. A small subgroup of Hashimoto’s thyroiditis is associated with IgG4-related disease. Virchows Arch. 2016; 468:321–7.
Article41. Koneczny I. Update on IgG4-mediated autoimmune diseases: new insights and new family members. Autoimmun Rev. 2020; 19:102646.
Article42. Wang H, Xu Q, Zhao C, Zhu Z, Zhu X, Zhou J, et al. An immune evasion mechanism with IgG4 playing an essential role in cancer and implication for immunotherapy. J Immunother Cancer. 2020; 8:e000661.
Article43. Bianchini R, Karagiannis SN, Jordakieva G, Jensen-Jarolim E. The role of IgG4 in the fine tuning of tolerance in IgE-mediated allergy and cancer. Int J Mol Sci. 2020; 21:5017.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrasonographic Findings of Papillary Thyroid Cancer with or without Hashimoto's Thyroiditis
- A case of Hashimoto's thyroiditis coexisting with thyroid papillary and follicular carcinoma
- Thyroid MALT Lymphoma Associated with Thyroid Papillary Cancer
- A Case of Subsequent Papillary Carcinoma of the Thyroid gland and Hashimoto's Thyroiditis
- Synchronous Occurrence of Papillary Thyroid Carcinoma and Mucosa-Associated Lymphoid Tissue Lymphoma: a Single Case Report