Endocrinol Metab.
2024 Jun;39(3):450-460. 10.3803/EnM.2023.1872.
Diagnostic Accuracy of Preoperative Radiologic Findings in Papillary Thyroid Microcarcinoma: Discrepancies with the Postoperative Pathologic Diagnosis and Implications for Clinical Outcomes
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Uijeongbu Eulji Medical Center, Eulji University, Uijeongbu, Korea
- 3Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 5Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 6Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Korea
- 7Genomic Medicine Institute, Medical Research Center, Seoul National University, Seoul, Korea
- KMID: 2556637
- DOI: http://doi.org/10.3803/EnM.2023.1872
Abstract
- Background
The diagnostic accuracy of preoperative radiologic findings in predicting the tumor characteristics and clinical outcomes of papillary thyroid microcarcinoma (PTMC) was evaluated across all risk groups.
Methods
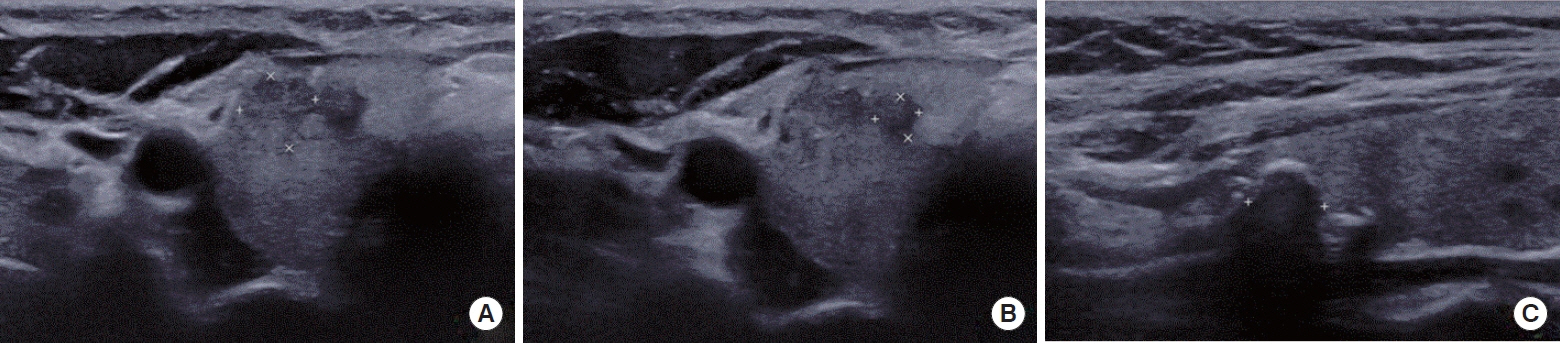
In total, 939 PTMC patients, comprising both low-risk and non-low-risk groups, who underwent surgery were enrolled. The preoperative tumor size and lymph node metastasis (LNM) were evaluated by ultrasonography within 6 months before surgery and compared with the postoperative pathologic findings. Discrepancies between the preoperative and postoperative tumor sizes were analyzed, and clinical outcomes were assessed.
Results
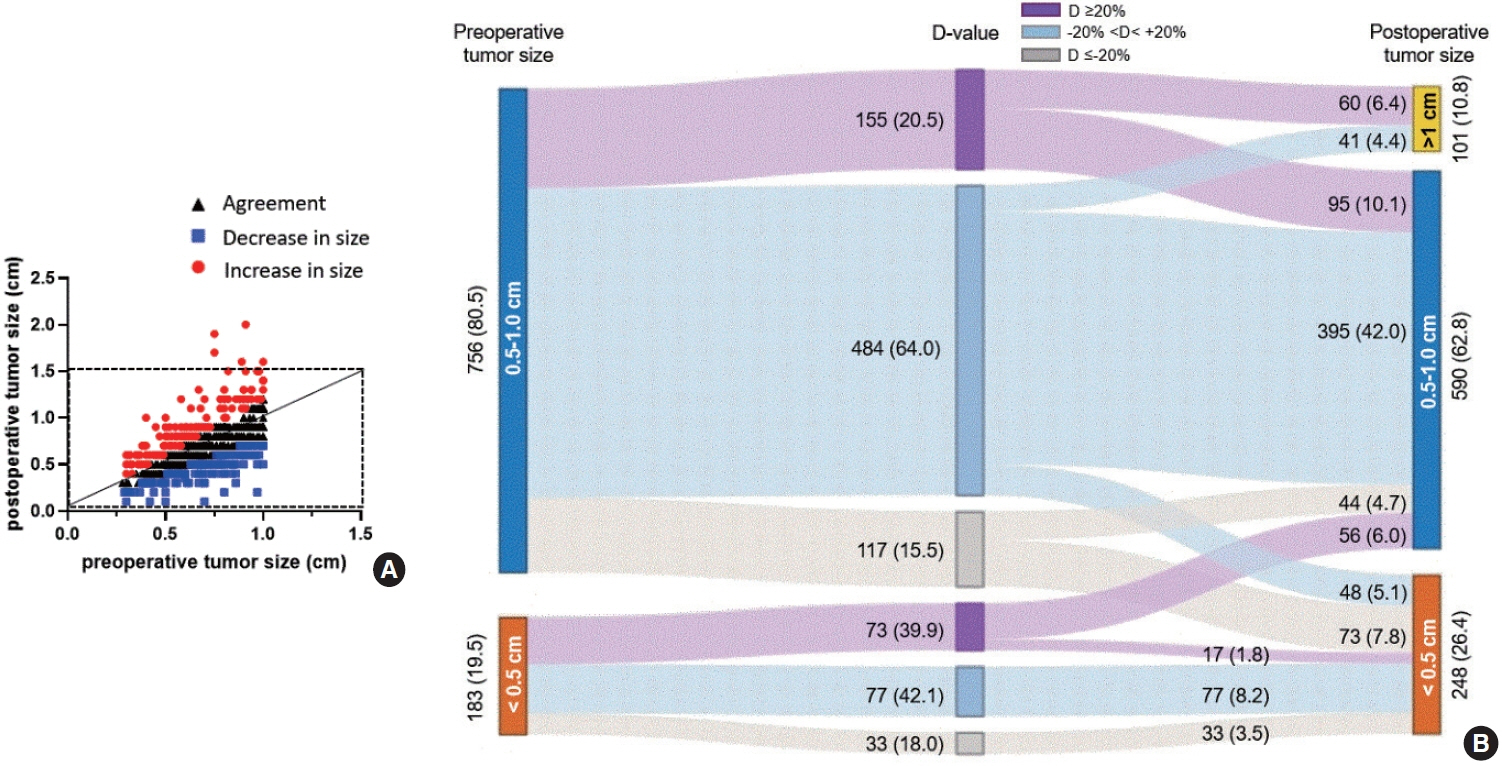
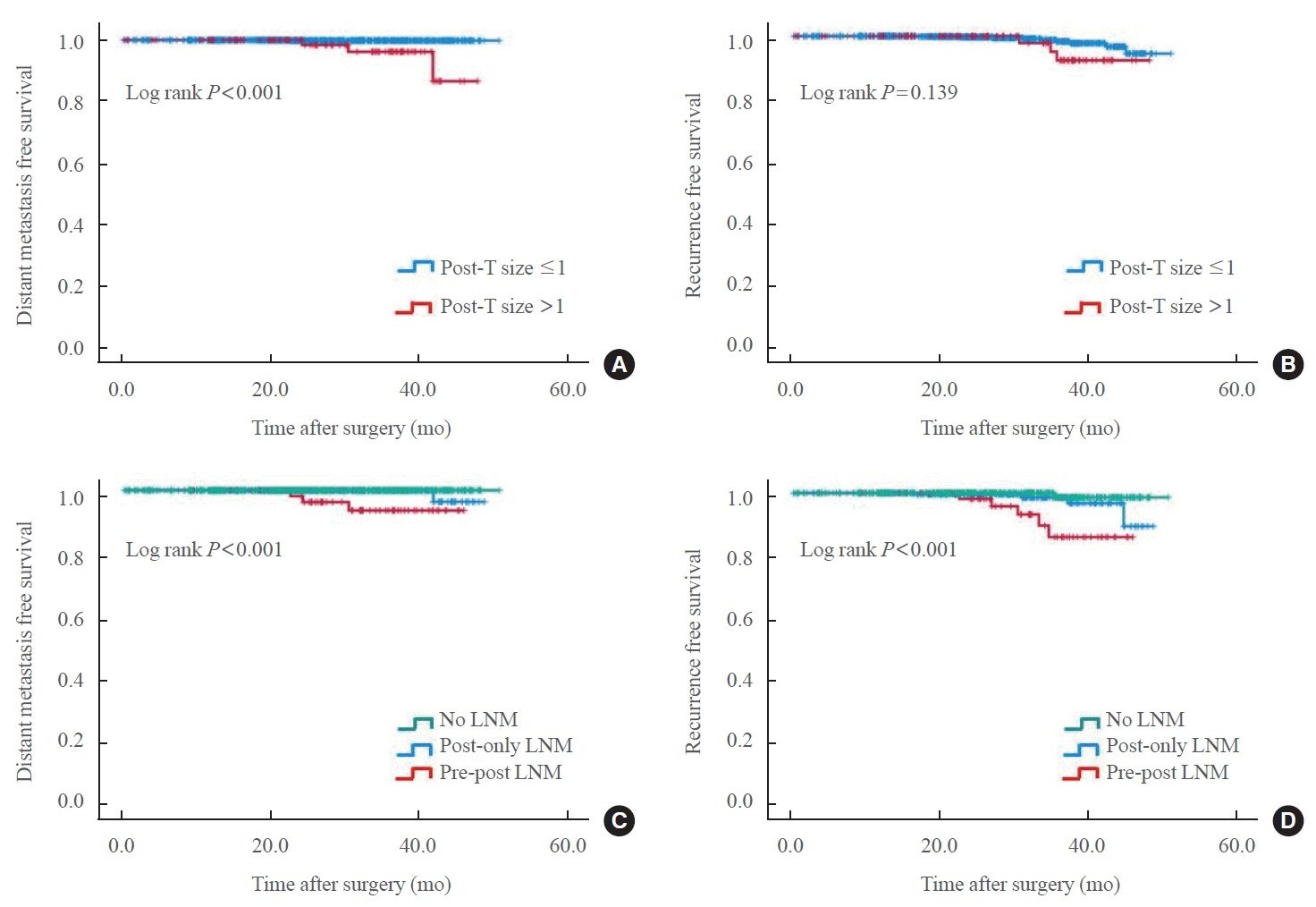
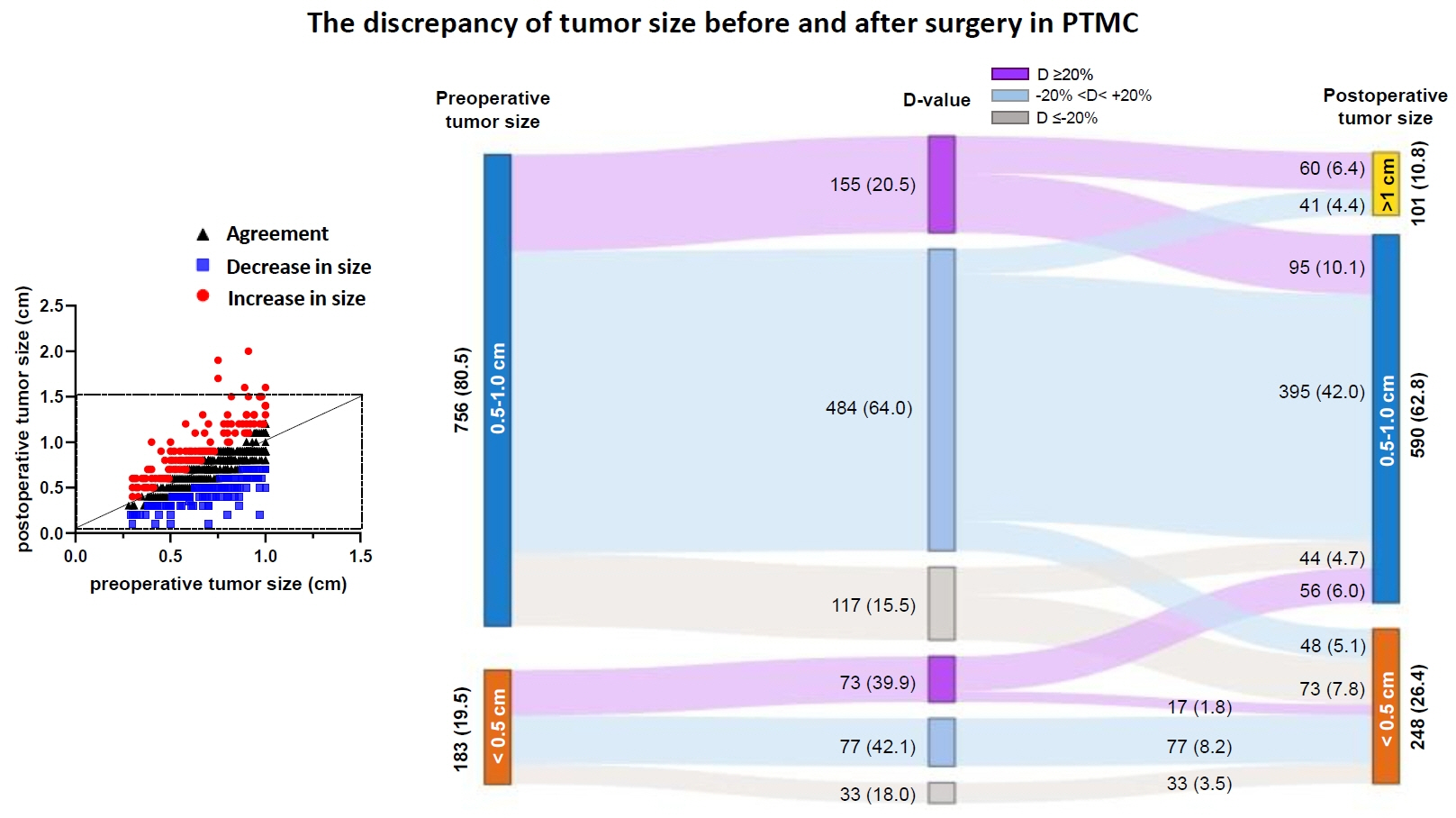
The agreement rate between radiological and pathological tumor size was approximately 60%. Significant discrepancies were noted, including an increase in tumor size in 24.3% of cases. Notably, in 10.8% of patients, the postoperative tumor size exceeded 1 cm, despite being initially classified as 0.5 to 1.0 cm based on preoperative imaging. A postoperative tumor size >1 cm was associated with aggressive pathologic factors such as multiplicity, microscopic extrathyroidal extension, and LNM, as well as a higher risk of distant metastasis. In 30.1% of patients, LNM was diagnosed after surgery despite not being suspected before the procedure. This group was characterized by smaller metastatic foci and lower risks of distant metastasis or recurrence than patients with LNM detected both before and after surgery.
Conclusion
Among all risk groups of PTMCs, a subset showed an increase in tumor size, reaching 1 cm after surgery. These cases require special consideration due to their association with adverse clinical outcomes, including an elevated risk of distant metastasis.
Keyword
Figure
Reference
-
1. Vigneri R, Malandrino P, Vigneri P. The changing epidemiology of thyroid cancer: why is incidence increasing? Curr Opin Oncol. 2015; 27:1–7.2. Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”: screening and overdiagnosis. N Engl J Med. 2014; 371:1765–7.
Article3. Park S, Oh CM, Cho H, Lee JY, Jung KW, Jun JK, et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ. 2016; 355:i5745.
Article4. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014; 140:317–22.
Article5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
Article6. Jeon MJ, Kim WG, Kim TY, Shong YK, Kim WB. Active surveillance as an effective management option for low-risk papillary thyroid microcarcinoma. Endocrinol Metab (Seoul). 2021; 36:717–24.
Article7. Loncar I, van Dijk SP, Metman MJ, Lin JF, Kruijff S, Peeters RP, et al. Active surveillance for papillary thyroid microcarcinoma in a population with restrictive diagnostic workup strategies. Thyroid. 2021; 31:1219–25.
Article8. Cho SJ, Suh CH, Baek JH, Chung SR, Choi YJ, Chung KW, et al. Active surveillance for small papillary thyroid cancer: a systematic review and meta-analysis. Thyroid. 2019; 29:1399–408.
Article9. Lee EK, Moon JH, Hwangbo Y, Ryu CH, Cho SW, Choi JY, et al. Progression of low-risk papillary thyroid microcarcinoma during active surveillance: interim analysis of a multicenter prospective cohort study of active surveillance on papillary thyroid microcarcinoma in Korea. Thyroid. 2022; 32:1328–36.
Article10. Gong Y, Li G, Lei J, You J, Jiang K, Li Z, et al. A favorable tumor size to define papillary thyroid microcarcinoma: an analysis of 1176 consecutive cases. Cancer Manag Res. 2018; 10:899–906.
Article11. Jeon MJ, Kim WG, Choi YM, Kwon H, Lee YM, Sung TY, et al. Features predictive of distant metastasis in papillary thyroid microcarcinomas. Thyroid. 2016; 26:161–8.
Article12. Wang X, Lei J, Wei T, Zhu J, Li Z. Clinicopathological characteristics and recurrence risk of papillary thyroid microcarcinoma in the elderly. Cancer Manag Res. 2019; 11:2371–7.13. Yi KH. The revised 2016 Korean Thyroid Association guidelines for thyroid nodules and cancers: differences from the 2015 American Thyroid Association guidelines. Endocrinol Metab (Seoul). 2016; 31:373–8.
Article14. Kang S, Lee E, Chung CW, Jang HN, Moon JH, Shin Y, et al. A beneficial role of computer-aided diagnosis system for less experienced physicians in the diagnosis of thyroid nodule on ultrasound. Sci Rep. 2021; 11:20448.
Article15. Hahn SY, Shin JH, Oh YL, Son YI. Discrepancies between the ultrasonographic and gross pathological size of papillary thyroid carcinomas. Ultrasonography. 2016; 35:220–5.
Article16. Ito Y, Kudo T, Kihara M, Takamura Y, Kobayashi K, Miya A, et al. Prognosis of low-risk papillary thyroid carcinoma patients: its relationship with the size of primary tumors. Endocr J. 2012; 59:119–25.
Article17. Ito Y, Fukushima M, Kihara M, Takamura Y, Kobayashi K, Miya A, et al. Investigation of the prognosis of patients with papillary thyroid carcinoma by tumor size. Endocr J. 2012; 59:457–64.
Article18. Bachar G, Buda I, Cohen M, Hadar T, Hilly O, Schwartz N, et al. Size discrepancy between sonographic and pathological evaluation of solitary papillary thyroid carcinoma. Eur J Radiol. 2013; 82:1899–903.
Article19. Yoon YH, Kwon KR, Kwak SY, Ryu KA, Choi B, Kim JM, et al. Tumor size measured by preoperative ultrasonography and postoperative pathologic examination in papillary thyroid carcinoma: relative differences according to size, calcification and coexisting thyroiditis. Eur Arch Otorhinolaryngol. 2014; 271:1235–9.
Article20. Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017; 143:1015–20.
Article21. Sanabria A. Experience with active surveillance of thyroid low-risk carcinoma in a developing country. Thyroid. 2020; 30:985–91.
Article22. Tuttle RM, Fagin J, Minkowitz G, Wong R, Roman B, Patel S, et al. Active surveillance of papillary thyroid cancer: frequency and time course of the six most common tumor volume kinetic patterns. Thyroid. 2022; 32:1337–45.
Article23. Gallo M, Pesenti M, Valcavi R. Ultrasound thyroid nodule measurements: the “gold standard” and its limitations in clinical decision making. Endocr Pract. 2003; 9:194–9.
Article24. Hegedus L. Thyroid size determined by ultrasound. Influence of physiological factors and non-thyroidal disease. Dan Med Bull. 1990; 37:249–63.25. Knudsen N, Bols B, Bulow I, Jorgensen T, Perrild H, Ovesen L, et al. Validation of ultrasonography of the thyroid gland for epidemiological purposes. Thyroid. 1999; 9:1069–74.
Article26. Deveci MS, Deveci G, LiVolsi VA, Gupta PK, Baloch ZW. Concordance between thyroid nodule sizes measured by ultrasound and gross pathology examination: effect on patient management. Diagn Cytopathol. 2007; 35:579–83.
Article27. Baloch ZW, LiVolsi VA. Post fine-needle aspiration histologic alterations of thyroid revisited. Am J Clin Pathol. 1999; 112:311–6.
Article28. Pandit AA, Vaideeswar P, Mohite JD. Infarction of a thyroid nodule after fine needle aspiration biopsy. Acta Cytol. 1998; 42:1307–9.29. Gordon DL, Flisak M, Fisher SG. Changes in thyroid nodule volume caused by fine-needle aspiration: a factor complicating the interpretation of the effect of thyrotropin suppression on nodule size. J Clin Endocrinol Metab. 1999; 84:4566–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Cystic Lymph Node Metastasis from Thyroid Papillary Microcarcinoma
- A Case of Ectopic Thyroid Papillary Carcinoma with Incidental Papillary Thyroid Microcarcinoma
- Papillary Thyroglossal Duct Cyst Carcinoma with Synchronous Occult Papillary Thyroid Microcarcinoma
- Multifocality and Bilaterality of Papillary Thyroid Microcarcinoma
- Role of Preoperative Staging Ultrasound for Papillary Thyroid Microcarcinoma